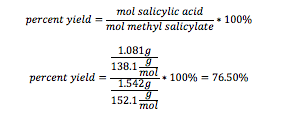Synthesis of Aspirin
Written by Katie
Introduction
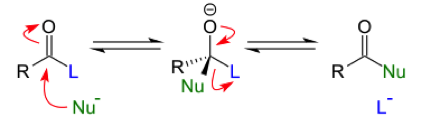
Methyl Salicylate is a naturally occurring chemical that is obtained from winter green oil that can be used to synthesize Salicylic Acid. Methyl Salicylate is a phenol with a acetyl group in the ortho position. This carbonyl group can undergo typical carbonyl addition-elimination reactions. An addition elimination reaction occurs at the carbonyl carbon where a nucleophile attacks the highly electrophilic carbonyl carbon. The carbonyl carbon is more electrophilic than the other carbons because the double bonded oxygen withdraws a great amount of the electron density making the carbon highly electropositive. After the nucleophile attacks the carbon, a tetrahedral intermediate with 4 substituents are coming off of the central carbon, one being an oxygen anion, is formed. Then the substituent that is the best leaving group leaves, and the carbonyl group is reformed.
|
|
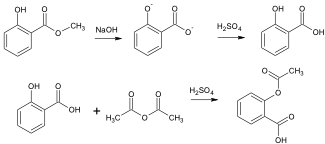
To synthesize salicylic acid, methyl salicylate (limiting reagent) is added to sodium hydroxide and heated. In this reaction the hydride ion acts as the nucleophile, attacking the carbonyl carbon and replacing the methoxy group. Once the reaction is complete, acid is added to the mixture to protonate the carboxylate ions to produce the acid. For the reaction to go reasonably fast and to completion, sodium hydroxide is added in excess to ensure the maximum amount of methyl salicylate reacts. Once Salicylic Acid (limiting reagent) is synthesized, it too can undergo a carbonyl addition-elimination with acetic anhydride to produce aspirin. This time the hydroxyl group of the salicylic acid acts as the nucleophile to attack the carbonyl group of the acetic anhydride and replace one of the acetyl groups.
|
|
After the synthesis of aspirin, the product was recrystallized for purification to rid the product of impurities. A major impurity that could be found in the crude product would be unreacted salicylic acid. The crude product and the purified product can be tested using ferric chloride. Ferric chloride forms highly colored complexes with phenolic compounds. So when the ferric chloride is added to the product, one can tell if the product mixture contains any of the starting material by the color of the solution. Solutions containing the phenolic group are dark purple and ones without are a yellow color. The two products synthesized in this lab also had their IR spectra and melting points taken for identification.
Data
Masses of Starting Material:
Methyl Salicylate:1.542g
Salicylic Acid: 2.087g
Mass of Products and Melting Points:
Salicylic Acid: 1.081g 154-155 degrees Celsius
Aspirin: 1.600g 134-135 degrees Celsius
Percent Yields:
Salicylic Acid: 76.50%
Aspirin: 58.75%
Ferric Chloride Test
Crude Product: yellow/orange
Aspirin: yellow/orange
Salicylic Acid: purple
Sample Calculations
Results
1.081g of Salicylic acid was obtained after the first synthesis. It was thin, short white crystals and had a melting point range or 154-155 degrees Celsius. 1.600g of aspirin was obtained after the second synthesis. This was a white powder and had a melting point range of 134-135 degrees Celsius.
Discussion
To confirm that the final product of the synthesis was aspirin, an IR spectrum of the product was taken. This spectrum showed a broad peak at 3220.11cm-1 which indicates that the product contained an –OH group. Hydroxyl groups tend to show characteristic peaks between 3300-2500 cm-1. This peak would have been broader if it wasn’t for the Nujol which was used to take the IR spectrum. The Nujol contains many hydrocarbons which produce a very strong peak in the 2900 range. The structure of aspirin also indicates that there should be a peak for a carbonyl ester, and a carbonyl acid. These peaks usually occur in the 1800-1715 cm-1 and 1725-1680 cm-1 range respectively. The spectrum showed a peak at 1717 cm-1 which indicated the ester carbonyl and a peak at 1687 cm-1 for the acid carbonyl. There are also smaller peaks which indicate the other carbon-oxygen groups of the aspirin. However, these lie in the finger print region of the spectrum and are therefore too hard to differentiate.
The melting points of both the salicylic acid and aspirin were taken as other means to identify, and test the purity of the products. Both the melting points of salicylic acid and aspirin were consistent with the literature values which are 159 degrees Celsius and 135 degrees Celsius respectively. This shows that both the products were relatively pure. The ferric chloride test was used to compare the salicylic acid, crude aspirin, and purified aspirin. After the addition of the ferric chloride, the salicylic acid solution turned purple, and both the aspirin solutions were yellow. Because both the aspirin solutions were yellow, there was not any unreacted starting material present in the product. If any was present in the crude or purified product it was not in a high enough concentration to complex with the ferric chloride to create the purple color.
The salicylic acid was obtained with 76.5% yield so there are sources of product loss. One possibility for loss of product could be during the acidification step. The synthesis of the salicylic acid is done by creating the anion of the acid which is soluble in the aqueous solution. It is then protonated to crash the salicylic acid out of solution. If not enough acid was added to protonate all the product, some will stay in the aqueous solution and will be lost. Another source of product loss is during the recrystallization. Because it is impossible to fully precipitate compound out of a solution, a small amount will always be loss.
The aspirin was obtained with 58.75%. One possible source of error is in the nature of the reaction of aspirin. These carbonyl reactions are all equilibrium reactions which means it is impossible for the reaction to go to 100% completion. Therefore during the synthesis of the aspirin some of the aspirin will be converted back to starting material. To help push the reaction toward the products, one could distill off the acetic acid. This is because the reduction of the products will cause more starting material to react to regain equilibrium. Another source of product loss was during the filtration of the product. The aspirin was small, powdery crystals. Since they were so small, some could have passed through the filter paper. To maximize yield, recrystallizing/refiltering the filtrate would be wise because more product could be collected. However, due to time constraints, refiltering could not happen.
Sources:
http://en.wikipedia.org/wiki/Nucleophilic_acyl_substitution