Synthesis of Salicylic Acid from Wintergreen Oil
Synthesis of Salicylic Acid from Wintergreen Oil
By: Matthew Rook
Introduction:
Experiment 3: Synthesis of Salicylic Acid from Wintergreen Oil
Purpose:
The purpose of this lab was to get students familiar with glass materials often used by organic chemists and how to reflux a mixture and to filter its precipitate, through teaching students how to create salicylic acid from methyl salicylate (wintergreen oil). During the experiment students learns how to use solubility data and then the students learns how to get melting points.
Mechanism:
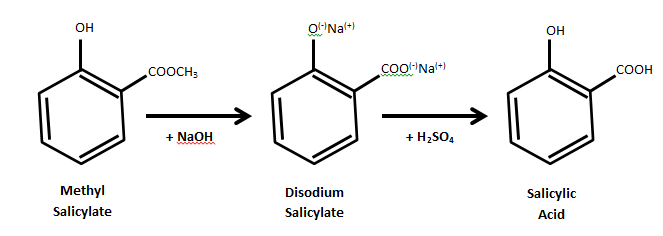
Procedure:
- Attached an air condenser to a conical vial.
- Measured 2 mmol of methyl salicylate into conical vial.
- Added 2.5 mL of 6M NaOH and some boiling chips.
- Heated reaction for 30 minutes after boiling started.
- Turned off burner when mixture was clear.
- Removed condenser when it was cool enough to touch, then waited till room temp.
- Transferred the mixture to a beaker, then slowly added H2SO4 until pH=2.
- Cooled the mixture in ice-water for 5 minutes.
- Let mixture settle and then vacuum filtered the mixture.
- Dissolved the solid by using a minimal amount of boiling hot water.
- Then cooled the solution to room temp, then cooled in ice bath for 8 minutes
- Vacuum filtered and measured the melting range of dry product.
Results:
| Compound | MW/M | mass/volume used/formed | Moles used/formed |
| Methyl Salicylate | 152.1 g/mol | 0.31 g | 0.002038 mol |
| Sodium Hydroxide | 6M | 2.5 mL | 0.015 mol |
| Sulfuric Acid | 3M | 2.4 mL | 0.0072 mol |
| Salicylic Acid | 138.1 g/mol | .60 g | 0.00434 mol |
| DI Water | X | 3.5 mL | X |
| Salicylic Acid (after recrystalization) | 138.1 g/mol | .16 g | 0.00116 mol |
Melting Point of the recrystallized salicylic acid: 125-130ᵒC
Theoretical Yield of Salicylic Acid (SA) (g):
(.002038 mol Methyl Salicylate)*(1 mol SA/1 mol Methyl Salicylate)*(138.1 g SA/1 mol SA)= .281 g Salicylic Acid
|
Percent Yield:
% Yield= (Actual yield)/(Theoretical Yield) * 100%
% Yield= (.16 g Salicylic Acid)/(.281 g Salicylic Acid) * 100%= 56.9% Yield
Discussion:
My yield for the production of Salicylic Acid was 56.9%. This was due majorly for the fact that the filteration system on many of the suction faucets did not work, making for repeated trials while losing parts of product in the process. My melting point for the recrystallized salicylic acid was in a range of 125-130ᵒC, which did not overlap the literature value of 159ᵒF. This may have been due to a failure to filter out by products and the use of too much water in the process.
Conclusion:
In the lab, it was apparent that all the students were able to use the air condenser and all other glassware used in the experiment. The students also learned how to create salicylic acid from methyl salicylate by the use of refluxing and filtration. During recrystallization and analysis, the students learned how to locate and utilize solubility data and also determine melting points. One change that should be made to this lab is to proportion everything in higher quantities, making it easier to get certain quantities of liquid and transfer it with the accurate amount present.
