Amine Addition to a Phenylalanine Derivative
Written by Jessie
Purpose:
The purpose of this experiment is to add an amine group to a Boc protected unnatural phenylalanine derivative (Amine Addition)
Results, Discussion, and Conclusions. (1 page maximum)
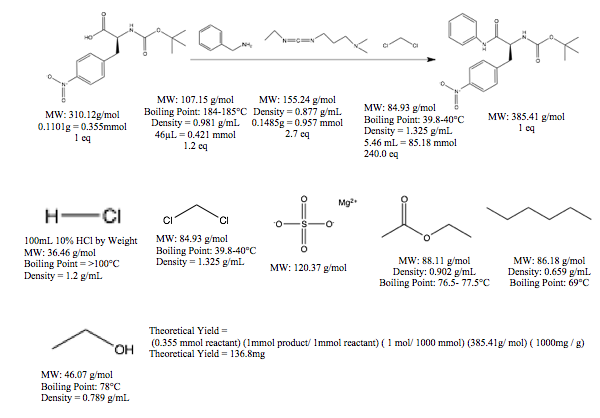
Sample Calculations:
Rf = Spot distance / solvent distance
Rf product = 3.97cm / 4.66cm = 0.852
Percent Yield = (41.1mg / 136.8mg) X 100% = 30.04%
In this reaction, the first step involved adding benzylamine to the reactant. This reagent is a necessary component to the reaction as it supplies the necessary atoms to add the desired amine to the reactant. Next, EDC was added to the reaction vial. This compound is a base used to deprotenate the acid reactant in the first step and then serve as a reagent that helps the reaction progress towards the products. Next, reaction grade dichloromethane was added to the reaction vial to serve as the neutral, nonreactive solvent. Additional, unwanted reactions will not occur in this solvent. Following storage of the reaction vial, which allowed the reaction to proceed to completion, the product was diluted with dichloromethane to halt any remaining reaction in solution. It was then acidified in 10% HCl in order to protonate the product and cause it to be soluble in the organic layer. An extraction procedure was performed in the HCl to remove any water-soluble impurities or remaining reagents from the reaction. The products, contained in the organic layer were dried in MgSO₄ in order to remove any remaining aqueous molecules. The collected product was run on a TLC plate in 2:1 ethyl acetate : hexanes in order to check for the presence of the desired product. It was run against the reactant on order to better determine whether the reaction went to completion. Once product presence was confirmed, the solvent was vacuumed out to remove any organic impurities, leaving only the product. Then, the product was redissolved in dichloromethane in order to continue with the purification process. The product was run through a silica column slurry containing 2:1 ethyl acetate : hexanes. However, the inability of this solvent to elute the product necessitated the use of ethanol, a more polar solvent to elute the product. The collected fractions were tested on a TLC plate in order to ensure that the product was eluting and then to determine if all of it had eluted. The collected fraction containing product were concentrated and again the solvent was vacuumed out in order to leave just the desired product in the vial.
The percent yield of this experiment, 30.04% is moderately low indicating that more care must be taken while performing the lab procedure. Some of the product could have escaped into the vacuum thus accounting for the low percent yield. The purity of this product cannot be confirmed because the reactant, Boc-Phe(pNO2)-OH did not resolve on the TLC. This could be due in part to making a solution too dilute, that not enough product was actually spotted on the TLC plate. Additionally, because no melting point or recrystallization data were taken, the purity of the compound is unknown. NMR or any other test should be performed to check the purity of the compound and to determine whether the desired product was actually synthesized.