Poprocks and Soda Lab Experiment
Written by Charisa
Abstract
In this experiment, the properties of the reaction of poprocks and soda will be observed. Using a graduated cylinder and a tub of water, the amount of CO2 released can be measured based on the ideal gas law formula. The graduated cylinder will be filled with water then placed upside-down in the tub of water, so that the gas escapes into the measurable graduated cylinder. In addition, the energy released from the reaction will be calculated with a given mass of poprocks, based on the formula to find q, which is the heat released from the reaction.
Procedure
Bluedoor. Candy and Soda Go Boom? Bluedoor Labs. 21 Oct. 2013. www.bluedoorlabs.com/online_manual
Part 2
|
Trial 1 |
Trial 2 |
Trial 3 |
|
22 Degrees C |
22 Degrees C |
22 Degrees C |
|
.506g Poprocks |
.509g Poprocks |
.503g poprocks |
|
110mL CO2* |
25.5mL CO2 |
36.0mL CO2 |
Calculations
Part 1: Calculate the heat released
q = m*C*(Final temp – Initial temp) Where: q = heat
m = mass of crushed poprocks (.508g)
C = specific heat of water (4.186J/g*C) Change in temp = 1 C
q= (.508g)*(4.186J/g*C)(1 C) q= 2.13J released.
Part 2 Calculate volume of CO2 Produced
to calculate moles of gas total, use PV=nRT where
P = atmospheric pressure (1atm)
V = Average volume of gas produced (.0572L) n = moles of total gas (being solved for)
R = .0821 (constant)
T = Temperature in K (295.15K)
(1atm)(.0572L) = n(.0821)(295.15K) n= .0024 mol total gas
To calculate Pressure of CO2:
Total pressure = Pressure of CO2 – Water Vapor pressure @ 22 Degrees C in atm (19.8mmHg/760mmHg = .026atm)
1atm = Pressure of CO2 – .026atm Pressure of CO2 = .974atm
To calculate Moles of CO2:
PCO2= Moles of CO2/Moles of total gas * Total pressure .974atm = Moles CO2/.0024mol *1atm
Moles CO2 = .0023mol
To calculate Volume of CO2:
Use PV = nRT arranged to solve for Volume
Volume of CO2 = Moles of CO2 * (.0821) *Temperature in K (295.15K) V = .0023mol * (.0821) * (295.15K)
V = .057L for .506g of poprocks
To calculate how much poprocks is dangerous to health, if 4L is the limit:
Set proportion to calculate g of poprocks
Conclusion
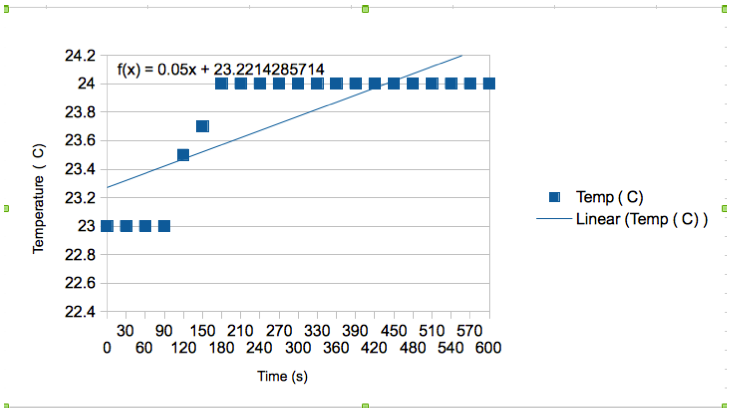
In conclusion, this experiemnt was a success in both parts. Using the formula to calculate heat of the exerthermic experiment, it could be calculated that 2.13J of energy is relased when .508g of poprocks in soda. After measure the temperature of the experiment every 30 seconds for 10 minutes, it could be concluded that the temperature just barely increased, with an overal change of 1degree. This shows that if one was to drink soda and have .5g of poprocks, it would not release a dangerous amount of energy. In the second part of the experiment, the volume of gas (CO2) was calculated to be .057L for .506g of poprocks. This number was calculated using the average of all three trials. One major error that occured was in trial one, where there was a plastic ending on the tubing. This caused the entire cylinder to fill with gas, therefore creating a very large volume. For the rest of the trials, the plastic tube was removed and the results came out normal. To combat this error, it is important to check the end of the tubing, and make sure it is consistent for all three trials.