Synthesis of Malachite and Verdigris
Synthesis of Malachite and Verdigris
By: Alyssa Brunal
OBJECTIVE
To perform synthetic reactions as provided in a primary journal article, provide a complete reference citation using EndNote, document observations of chemical reactions, determine if data is quantitative or qualitative, and separate a precipitate using gravity and vacuum filtration.
EXPERIMENTAL METHOD
Materials
All materials required for this experiment were provided by the Virginia Tech Department of Chemistry.
Synthesis
MALACHITE
Part A: Creation of Malachite Mixture
The Malachite mixture was made by adding 3.10g of CuSO4.5H2O to 12.5 mL of distilled water, which was then stirred using a stir plate. A second solution of 1.45g of Na2CO3 and 25mL of Distilled water was then added to the original mixture slowly with constant stirring. The new mixture was then placed, while still in the beaker, into a 400 mL beaker filled with an ice bath for 30 minutes.
Part B: Gravity Filtration
The solution was then poured into a funnel lined with filter paper placed in a flask and sat for 20 minutes. Distilled water was used to ensure that no solid was sticking to the sides of the beaker or funnel. The precipitate was then left in the top of the funnel with the liquid in the waste flask.
VERDIGRIS
Part A: Creation of Verdigris Solution
4.2g of CuSO4.5H2O was added to 50 mL of water in a 250mL beaker then stirred vigorously. 100mL of ammonia was added to a 100 mL beaker and the transferred via pipet into the previous solution, drop by drop until the solution turned a deep blue color. (Between 25mL and 35mL was added). This mixture was stirred for 5 minutes and then sent through vacuum filtration, creating a vacuum in a flask with running water then using a Buchner funnel with filter paper to place the solution and remove the liquid from the precipitate.
Part B: Addition of Sodium Hydroxide
After the vacuum filtration was completed we mixed the damp precipitate with 40mL of distilled water and stirred until we finished then adding 8.5mL of 2.0M sodium hydroxide. We then sent the solution through vacuum filtration for a second time.
Part C: Observation of Verdigris Crystals
We then removed the precipitate, while keeping it on the filter paper, and dried it with another piece of filter paper. We added 40mL of Vinegar to a 250mL beaker and then added the precipitate. The mixture was then stirred for 4 minutes and took observations.
EXPERIMENTAL DATA
No data was recorded over the course of the lab.
OBSERVATIONS
Malachite
After making the malachite mixture, the solution was a light blue and very thin. After icing the solution for 30 minutes, the color remained the same however, the consistency was a lot thicker. After filtration, we were left with a clear liquid in the flask and a bright blue precipitate on the filter paper in the funnel.
Verdigris
After creating the first solution, we had a color similar to the Malachite solution. After adding the ammonia, the solution continued to darken until we saw a deep blue that was almost purple. The consistency of the solution was very watery and thin. After the first vacuum filtration, our liquid remained dark blue, but we had a bright light blue precipitate on the filter paper. We then added the water and sodium hydroxide which did not do anything to the color of our precipitate, but after preforming vacuum filtration for a second time our precipitate color brightened even more. After removing the filter paper, our precipitate’s color began to darken rapidly. After placing the precipitate into the vinegar, the precipitate began to dissolve, and formed a blue-ish green solution in our beaker.
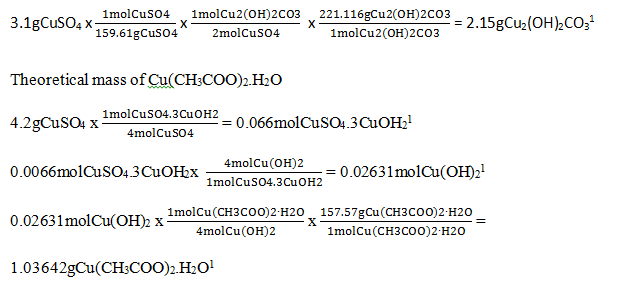
SAMPLE CALCULATIONS
The calculations below were used to draw various conclusions based on observations made during the lab.
Theoretical mass of Cu2(OH)2CO3(s)
RESULTS AND DISCUSSION
Malachite
Malachite is synthesized using the following reaction:
2CuSO4·5H2O(aq) + 2Na2CO3(aq) à CuCO3Cu(OH)2(s) + 2NA2SO4(aq) + CO2 + 9H2O
This demonstrates that when 2 moles of hydrated copper (II) sulfide reacts with aqueous sodium carbonate, a precipitate of copper (II) carbonate hydroxide. From this reaction one is able to separate the precipitate from the solution via gravity filtration, in which filter paper is used to allow liquid to pass through while keeping solid from reaching the collection flask. This solid, a pigment that is typically used in paints, can then be processed further to add color to various paints.2
Verdigris
The first step of the synthesis of Verdigris is shown in the following reaction:
4CuSO4·5H2O(aq) +6NH3(aq) à CuSO4·3Cu(OH)2(s) + 2(NH4)2SO4(aq) + 14H2O(l)2
In this step 4 moles of hydrated copper sulfate reacts with 6 moles of Ammonia to produce a precipitate of copper sulfide tribasic. This precipitate is then used in the next step, which is represented by the following reaction:
CuSO4·3Cu(OH)2(s) + 2NaOH(aq) à 4Cu(OH)2(s) + (Na)2SO4(aq)2
In this reaction the copper sulfide tribasic, the precipitate from the previous reaction, is then mixed with sodium hydroxide to produce a new precipitate of copper hydroxide. This new precipitate is require for the final step of verdigris synthesis. This step is represented by the following equation:2
Cu(OH)2(s) + 2 CH3COOH(aq) à Cu(CH3COOH)·H2O(aq) +H2O(l)2
The copper hydroxide from the previous reaction is mixed with acetic acid (vinegar) to then form the final solution. From this solution, one may then let evaporation take place over the course of about a week to then reveal the crystals of verdigris. The verdigris may then be processed even further to be used as a pigment is various thing such as paint. 2
EXPERIMENTAL UNCERTANTY
The instruments used in this experiment have various uncertainties. The balance used has an uncertainty of +/- 0.013, the graduated cylinder has an uncertainty of +/- 0.013, and the pipet has an uncertainty of +/- 0.013. Using these instruments and receiving an inaccurate reading could have affected our experiment at any point, altering the about of each chemical used or produced.
POST-LABORATORY QUESTIONS
1. It is important to wash the Cupric Hydroxide intermediate in the synthesis of Verdigris because it removes the Sodium and Sulfate from the precipitate leaving you with only Copper Hydroxide.
2. It is less difficult to precipitate Malachite than it is to precipitate verdigris because there is a two chemical combination to produce malachite while there is a two chemical combination to produce a precipitate which then can be added to another chemical to produce verdigris. There is no direct chemical reaction to produce verdigris.
3. Blue Copper Sulfate pentahydrate could be used as a pigment for making paint. (It was until about 3000 B.C.E.) It was replace however is because the compound is highly soluble and if it were to get wet, or interact with semi-humid air, the pigment would run. (Also Malachite and Verdigris came in around that time)
4. We had more of the verdigris solution, and with gravity filtration, the time it would have taken to filter out the precipitate twice with gravity filtration would have been way too long, vacuum filtration took a lot less time.
CONCLUSION
In the synthesis of malachite, hydrated copper (II) sulfate was mixed with aqueous sodium carbonate to create copper (II) carbonate hydroxide (malachite) in solution which was then filtered to separate the precipitate. In the synthesis of verdigris, copper (II) sulfate was mixed with ammonia to create copper sulfide tribasic, which was then mixed with sodium hydroxide to produce copper hydroxide, which was finally mixed with acetic acid (vinegar) to produce verdigris solution. The verdigris solution would then be left out for a week to allow evaporation to occur and for the verdigris crystals to form.
REFERENCES
1. CRC Handbook of Chemistry and Physics. 92nd Edition ed.; CRC Press: Boca Raton, FL. http://www.hbcpnetbase.com.ezproxy.lib.vt.edu:8080/ (accessed October 2013).
2. Sally D. Solomon, S. A. R., Megan L. Mahon, Erica M. Halpern, Synthesis of Copper Pigments, Malachite and Verdigris: Making Tempura Paint. 2011.
3. Patricia Amateis, V. L., Michelle Dalton, General Chemistry 1045. Hayden-McNeil 2014.
