The Synthesis of N,N-diethyl-m-toluamide (DEET) with Thiosemicarbazides as Potential Antimalarial Agents
Written by Alexis
Acknowledgements
I would like to thank the Department of Chemistry and Biochemistry for providing me with the necessary instruments that were needed for my study. I would like to thank Dr. Alvin A. Holder for allowing me to participate in his research lab and for working diligently alongside me. I would also like to thank the students of the Holder Research Lab for their involvement in the lab and for helping to ease along the progression of our spring semester together. In addition, I would like to acknowledge the NSF-REU grant that allowed me to participate in this prestigious research opportunity.
1.0 Introduction
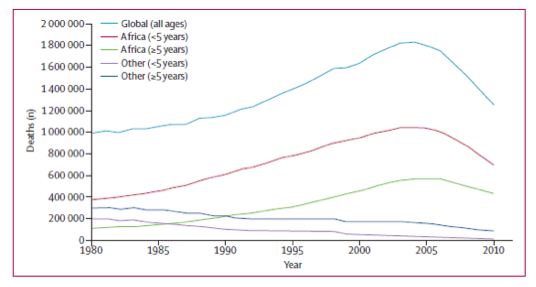
Malaria is a fatal, infectious disease which is caused by the transmission of the parasite, Plasmodium falciparum, via mosquitoes into the blood stream of mammalian hosts, especially humans. As a result of developing malaria, due to infection by the parasite, the host may experience neurocognitive impairment, anemia, or even death. Unfortunately, the rate of infection with malaria amongst humans has been steadily increasing each year. According to several studies, the contraction of malaria is most common in sub-Saharan Africa. In 2010, approximately 655,000 people died of malaria, most of which were young children of sub- Saharan Africa. Yet, malaria also affects the United States; there are approximately 1,500 cases of malaria diagnosed each year.
Figure 1. Trends in global malaria deaths by age and geographical region, 1980-2010 (Murray et. al)1
The severity of malaria and its increasing death rate have caused worldwide attention and the initiation of efforts to inhibit the disease. As a result of the widespread epidemic of malaria, several studies have been done on the disease and medicines have been developed. Currently, the most commonly used drugs to treat malaria are chloroquine and sulfadoxine-pyrimethamine. Though studies have shown that these drugs have been effective in the treatment of malaria, recently, P. falciparum has become resistant to both chloroquine and sulfadoxine-pyrimethamine. 2-3 Therefore, scientists have been working to produce new and improved compounds in order to potentially suppress or inhibit the transmission of the disease.
Thiosemicarbazones are essentially Schiff bases that are the result of the condensation of an aldehyde or a ketone with a thiosemicarbazide. 4 Currently, thiosemicarbazones are used in a variety of medical practices such as pharmacology and nuclear medicine. 5 Studies have shown that thiosemicarbazones, in combination with metal complexes, may be the solution to the issue of the epidemic of malaria considering that this class contains characteristics of anti-viral, anti-HIV, and tuberculostatic activity. 6 Yet, only a few these ligands have been reported to have antimalarial qualities due to their metal complexes. 7
Past studies have shown that thiosemicarbazones in combination with metals, such as gallium, have been used as therapeutic drugs. Since gallium possesses characteristics that resemble iron, it is able to be used to interact with the cellular processes of the body without being rejected by the body. As a result, gallium has been used to effectively treat a number of disorders including some cancers and infectious diseases. Notably, gallium has not been found to be immunosuppressive; this increases the potential for gallium to be used as a successful agent in the development of more therapeutic drugs.
Gallium-based complexes such as gallium nitrate (Ga[(NO3)3]) have been used to therapeutically treat hyperkalemia. Additionally, gallium nitrate has been found to effectively eliminate pain and inflammation. Clinical trials reported that when 14% aqueous solution of gallium nitrate was administered to a patient whose hands suffered from arthritis, the patient’s pain and inflammation was discontinued. The use of 40% gallium nitrate to treat frozen shoulder resulted in nearly complete elimination of pain along with complete restoration of motion.
The effective use of gallium within the body requires bioactivation. In order to bioactivate gallium, thiosemicarbazones, which contain antimicrobial properties, have been reacted with gallium complexes to form one compound.
Recently, a study on gallium and its affects when combined with thiosemicarbazones was conducted by Lessa et al. Several compounds were developed using 2-acetylpyridine, 2- formylpyridine, and 2-benzylpyridine-derived thiosemicarbazones in combination with gallium complexes. These compounds were analyzed in regard to potential antimalarial, antitumor, and antimicrobial activity. Specifically, GalliumprotoporphyrinIX (GaPPIX), a compound, was developed and assessed. GaPPIX presented in vitro growth-inhibitory activity against several gram-positive and gram-negative bacteria pathogenic bacteria. Notably, GaPPIX presented growth-inhibitory activity against the malarial parasite, P. falciparum. 8
Due to the results of previous studies gallium in combination with thiosemicarbazones and the implication of the effectiveness of gallium nitrate, we decided to use this compound to conduct one of our syntheses. Though the product of our synthesis has yet to be biologically assessed, it is plausible to predict that the use of gallium nitrate will aid in the effectiveness of the therapeutic aspects of the product.
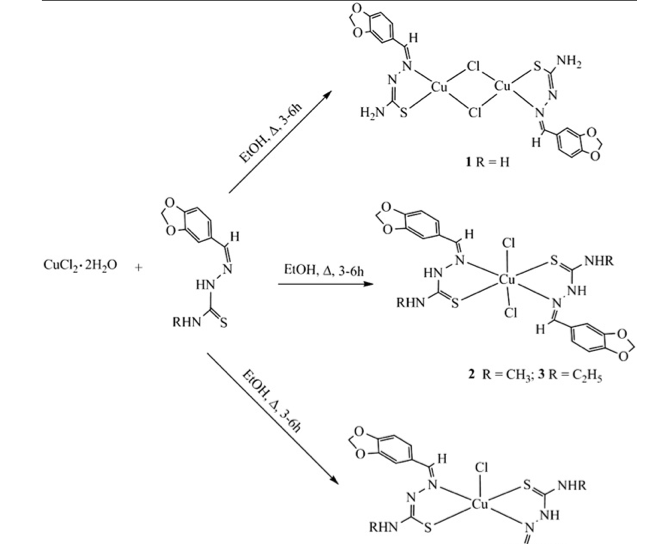
Within the past two years, it has been reported that Copper(II) thiosemicarbazone complexes have been assessed for effectiveness as a metallodrug in a variety of medical applications. In an effort to produce metallodrugs that will effectively aid in eliminating illnesses such as cancer, Beckford et al. conducted a study on the effectiveness of Copper(II) complexes in combination with thiosemicarbazones. As a result of the work pioneered by Beckford et al., several copper(II) complexes were synthesized from benzo[d][1,3]dioxole-5- carbaldehyde (piperonal) thiosemicarbazones. Elemental analysis and infrared spectroscopy was used to evaluate the complexes. The geometries of the complexes were based on the nature of the thiosemicarbazone that was used during each reaction. The products of these reactions including a dinuclear complex [Cu(HpTSC)Cl]2, a mononuclear complex [Cu(RHpTSC)2Cl2] (R=CH3 or C2H5) and another mononuclear complex [Cu(PhHpTSC)(PhpTSC)Cl]. These complexes presented the ability to strongly bind to human serum albumin (HSA), a protein that is significant to the transport of drugs through the body.
According to studies, copper(II) complexes in combination with thiosemicarbazones possess excellent chelating ability to HSA and have been found to be acceptable aids in inhibiting some cancer lines. 9 In order to assess the properties of copper(II) in regards to antimalarial treatment, we decided to develop a copper(II) complex using (E)-2- ((Diethylamino)(m-tolyl)methylene)-N-ethylhydrazinecarbothioamide (4-ethyl-3- thiosemicarbazideDEETTSC).
DEET or N,N-diethyl-m-methylbenzamide has been a primary component of our synthesis . DEET is a topical insect repellent that is widely used to combat mosquitoes, fleas, ticks, flies, and other biting insects in an effort to help prevent the transmission of arthropod-borne illnesses such as malaria and West Nile virus. DEET has been used in numerous commercial repellents, such as S.C. Johnson Off! Botanicals, which is a spray. Other commercial DEET formulations include lotions, sticks, soaps, gels, and impregnated towlettes. Insect repellents, containing either low or high concentrations of DEET are applied to the skin of humans and animals to help protect them from being bitten. DEET has been used for decades and has shown to be highly effective in controlling the biting rates of insects; also, it is generally safe for use. Since DEET has been effective in decreasing the risk for contracting malaria and other arthropod-borne illnesses, it is plausible to conclude that the combination of metals, thiosemicarbazones, and DEET will result in a successful agent against the spread of malaria. 10- 11
Though malaria is a severe disease that leads to death if untreated, there are scientists that are working diligently to aid in the prevention of malaria. 3 Due to our passion to aid saving the lives of people who have contracted malaria or are at risk of infection via the malarial parasite, P. falciparum, we have strived to synthesize compounds that will potentially not only suppress, but inhibit the progression of malaria.
2.0 Experimental
2.1 Materials
Reagents that were obtained from Sigma Aldrich were utilized for the preparation of several compounds. All of the chemicals that were used were provided in the lab by Dr. Holder. The Department of Chemistry and Biochemistry at the University of Southern Mississippi provided necessary instrumentation so that the compounds produced could be thoroughly analyzed.
2.2 Instrumentation
The Nicolet Nexus 410 FT-IR was used to collect IR spectra in order to analyze the compounds that were produced. 1H NMR and 13C NMR were acquired on a 400 MHz NMR Spectrometer. TLCs were conducted in order to evaluate the separation of the fractions of DEET and the reaction mixtures of DEET with TSCs when placed in silica gel.
2.3 Synthesis of DEET with 4-ethyl-3-thiosemicarbazide
DEET (2.39g, 2.395 ml, 12.495 mmol) was placed in 100 ml RB flask followed by anhydrous methanol (150 ml) and 4-ethyl-3-thiosemicarbazide (1.49g, 12.495 mmol) and five drops of hydrochloric acid. The reaction mixture was refluxed for 24 hours and then dried using rotary evaporation. After drying, a yield of 3.23g (93%) product was collected.
A TLC was conducted on the liquid DEET and the reaction mixture. A ratio of ethyl acetate (160 ml) and methanol (40 ml) was used for the solvent. The liquid DEET was labeled “R” and the reaction mixture was labeled “RM”.
The reaction mixture was kept at room temperature for approximately 16 hours.
A silica gel column was formed first using a 2:1 mixture of chloroform: ethyl acetate (400 ml chloroform, 200 ml ethyl acetate). A small amount of solvent mixture was used to dissolve the DEET with 4-ethyl-3-thiosemicarbazide and added to the column using a Pasteur pipette and added along the sides of the column. Silica gel (Sigma-Aldrich #288624-1KG) was used to make the column. The silica gel residue on the sides of the column was washed down using the solvent mixture. Fractions were collected from the column ~25 ml at a time (20 fractions were collected). It was predicted that the silica gel column should run off the impurities in the DEET 97% first.
A TLC was conducted on each of the fractions.
After fraction #75 was collected, methanol (100%) was added to the silica gel column.
2.4 Synthesis of DEET with 4-phenyl-3-thiosemicarbazide
DEET (2, 395 ul) was placed in a 100 ml RB flask followed by anhydrous methanol (50 ml) and 4-phenyl-3-thiosemicarbazide (12.495 mmol, 2.09 g) and five drops of hydrochloric acid. The reaction mixture was refluxed for 24 hours and filtered. Additionally, the reaction mixture was dried using rotary evaporation.
The reaction mixture was refrigerated for ~24 hours at -20C.
DEET (4.81 g, 4.822 ml, 25.152 mmol) was placed in a 250 ml RB flask followed by anhydrous methanol (100 ml) and 4-phenyl-3-thiosemicarbazide (3.00 g, 25.152 mmol) and five drops of concentrated hydrochloric acid. The reaction mixture was refluxed for 24 hours and then dried using rotary evaporation. The product was a thick, brown oil.
A TLC was conducted on DEET with 4-phenyl-3-thiosemicarbazide with a 2:1 mixture of chloroform: ethyl acetate and placed in chloroform solvent (100%) and then placed in methanol solvent (100%).
2.5 Comparison of TLCs of DEET with 4-ethyl-3-thiosemicarbazide and DEET with 4- phenyl-3-thiosemicarbazide
A TLC was conducted on both DEET with 4-phenyl-3-thiosemicarbazide and DEET with 4- ethyl-3-thiosemicarbazide and DEET as a control. The solvent used for the TLC was a 2:1 mixture of chloroform: ethyl acetate.
A second TLC was conducted on DEET (“R”) and DEET with 4-ethyl-3-thiosemicarbazide (“RM”) using a 4:1 mixture of ethyl acetate: methanol and then using a 2:1 mixture of chloroform: ethyl acetate. The Rf values of DEET and DEET with 4-ethyl-3-thiosemicarbazide were calculated and recorded.
2.6 Analysis of DEET, 4-ethyl-3-thiosemicarbazide, and DEET with 4-ethyl-3- thiosemicarbazide using 1H-NMR/ FT-IR spectroscopy
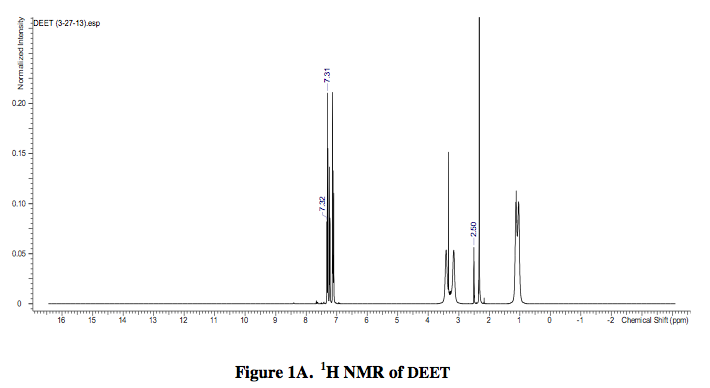
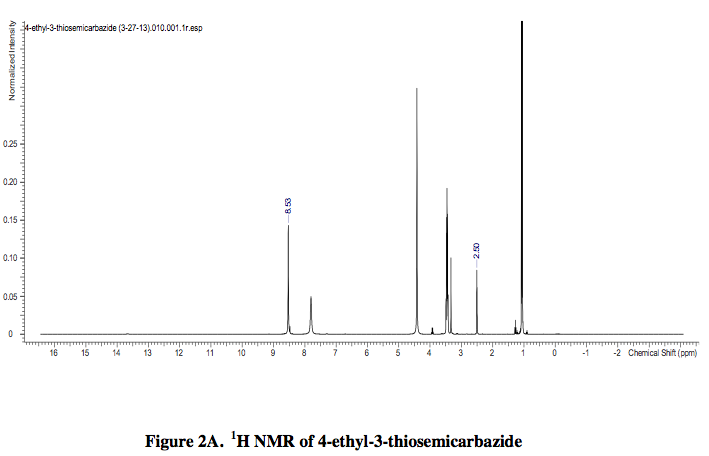
A 1H NMR spectra of DEET in DMSO-d6 was acquired as well as a 1H NMR spectra of 4-ethyl- 3-thiosemicarbazide in DMSO-d6 solvent.
A 1H NMR and FT-IR of 4-ethyl-3-thiosemicarbazide with DEET was obtained. DMSO-d6 was the solvent used to acquire the 1H NMR spectrum.
An FT-IR spectrum of DEET was also obtained.
2.7 Synthesis of [Ga(DEET TSC)2](NO3)3
(E)-2-((Diethylamino)(m-tolyl)methylene)-N-ethylhydrazinecarbothioamide (4-ethyl-3- thiosemicarbazideDEETTSC) (0.225g, 0.771mmol) and Ga(NO3)3x(H2O (0.092g, 0.355mmol) were mixed in a 100ml RB flask, followed by methanol (30ml). The reaction mixture was refluxed for 22 hours.
Rotary evaporation was used to dry the reaction mixture, resulting in yellow oil. A TLC was then done on the reaction mixture and 4-ethyl-3- thiosemicarbazide with DEET using the 2:1 mixture of chloroform: ethyl acetate as the solvent. Also, DEET was added to the TLC to compare the DEET to the 4-ethyl-3-thiosemicarbazide with DEET and the [Ga(DEET TSC)2] (NO3)3.
2.8 Synthesis of (E)-2-((Diethylamino)(m-tolyl)methylene)-N- ethylhydrazinecarbothioamide with Copper(II)Chloride
The synthesis of (E)-2-((Diethylamino)(m-tolyl)methylene)-N-ethylhydrazinecarbothioamide with Copper(II)Chloride was adapted from a procedure described by Beckford et al. 9
4-ethyl-3-thiosemicarbazideDEETTSC (0.298g, 1.02mmol) was added to a 100ml RB flask, followed by ethanol (20ml). Copper(II)Chloride (0.069g, 0.51mmol) dissolved in ethanol (10ml) was added slowly. The reaction mixture was refluxed for approximately 4 hours.
The reaction mixture was cooled after being refluxed and then filtered. A solid precipitate was formed and was collected. The solid was washed with ethanol and then with ether. Afterward, the solid was filtered on a vacuum and then air dried.
Copper(II) complex was added into three separate tubes. Three different solvents were added amongst the tubes. A sonicator was used to sonicate each of the mixtures that were developed to determine which solvent the copper(II) complex would be most soluble in. Each of the tubes was observed after sonication.
3.0 Results and Discussion
2.3.1. Synthesis of DEET with 4-ethyl-3-thiosemicarbazide
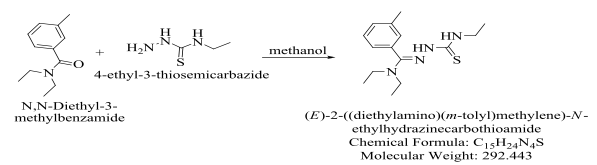
Scheme 1.
The synthesis of DEET with 4-ethyl-3-thiosemicarbazide required for the C=O on DEET to be reduced. The use of methanol caused the C=O to be reduced. The reduction of the C=O allowed for the DEET and the 4-ethyl-3-thiosemicarbazide to react in order to produce the product, formally named (E)-2-((diethylamino) (m-tolyl)methylene)-N-ethylhydrazinecarbothioamide. Also, used in the reaction was hydrochloric acid; this was used as a catalyst to drive the reaction forward into completion.
After the reaction mixture was dried, it was observed that the reaction mixture had produced a thick, yellow oil as the product.
After being kept at room temperature for approximately 16 hours, the reaction mixture was found to have formed a white solid. The appearance of oil could not be seen; yet the product looked similar to petroleum jelly.
Compound A. DEET with 4-ethyl-3-thiosemicarbazide
As the product was being further assessed using a silica gel column, it was noticed that the methanol (100%) was added to the column too soon resulting in the appearance of DEET on the TLC columns that were conducted.
An FT-IR spectrum of (E)-2-((diethylamino) (m-tolyl)methylene)-N- ethylhydrazinecarbothioamide was obtained and compared to the FT-IR peaks of spectra described by Lewis et al. 5 This comparison is outlined in Table 1.
2.4.1. Synthesis of DEET with 4-phenyl-3-thiosemicarbazide
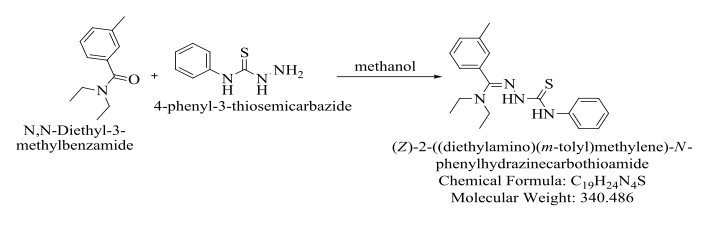
Scheme 2.
As with the synthesis of DEET with 4-ethyl-3-thiosemicarbazide, the synthesis of DEET with 4-phenyl-3-thiosemicarbazide required for the C=O bond on DEET to be reduced. The reduction of C=O was due to the use of methanol. The reduction of the C=O bond allowed the DEET and 4-phenyl-3-thiosemicarbazide to react and form the product, formally named (Z)-2-((diethylamino)(m-tolyl)methylene)-N- phenylhydrazinecarbothioamide. Hydrochloric acid was used to catalyze the reaction and drive it towards completion.
At the completion of the reaction, a white, solid precipitate was filtered. Rotary evaporation resulted in a colorless, sticky product. During refrigeration, the reaction mixture formed a cluster of white crystals; yet, the mixture still contained some oil. The synthesis was conducted a second time using more DEET and 4-phenyl-3- thiosemicarbazide under the same conditions as the first synthesis. The product of this synthesis was a thick, brown oil.
Larger amounts of DEET and 4-phenyl-3-thiosemicarbazide were used for the second synthesis, but five drops of hydrochloric acid was used to catalyze each reaction. Though it was not observed, it is plausible to conclude that the first synthesis occurred faster than the second synthesis due to the ratio of reactants to reagents.
2.5.1. Comparison of TLCs of DEET with 4-ethyl-3-thiosemicarbazide and DEET with 4-phenyl-3-thiosemicarbazide
The Rf value of DEET (“R”) was 0.545 cm and the Rf value of DEET with 4-ethyl-3- thiosemicarbazide was 0.473 cm.
2.6.1. Analysis of DEET and DEET with 4-ethyl-3-thiosemicarbazide using 1H NMR and FT-IR spectroscopy
The analysis of the FT-IR spectra of DEET and DEET with 4-ethyl-3-thiosemicarbazide that were obtained provided information on the functional groups that are present within each of the structures.
Relevant DEET peaks were at 2971.06 cm-1 and 1625.32 cm-1. Relevant peaks for 4- ethyl-3-thiosemicarbazide with DEET were 3244.50 cm-1, 2971.06 cm-1, 1533.44 cm-1, and 1636.39 cm-1.
It seems that there was a distinct peak that represents DEET at 2971.06 cm-1.
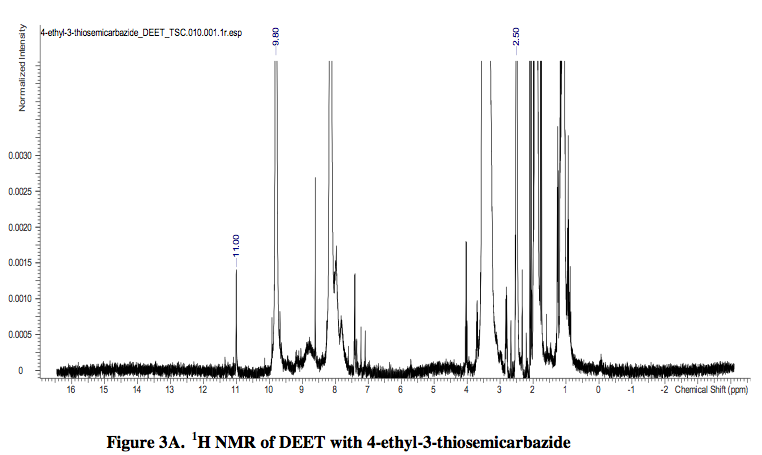
The analysis of the 1H NMR spectra of DEET, 4-ethyl-3-thiosemicarbazide, and DEET with 4-ethyl-3-thiosemicarbazide revealed that each contained a specific peak at 2.50 ppm. In the 1H NMR spectra of DEET and 4-ethyl-3-thiosemicarbazide, the peak at 2.50 ppm was weak. Yet, when the DEET and 4-ethyl-3-thiosemicarbazide were combined to form the product, (E)-2-((diethylamino) (m-tolyl)methylene)-N- ethylhydrazinecarbothioamide, the peak at 2.50 ppm was strong. Therefore, it is plausible to conclude that the peak at 2.50 ppm may be important to the structure of the product and perhaps its potency if used as an antimalarial drug.
2.7.1. Synthesis of [Ga(DEET TSC)2](NO3)3
[Ga(DEET TSC)2] (NO3)3 decomposed on the TLC whereas the DEET made one consistent spot (not decomposing). This would be the same result as if this were done on a silica gel column. The product would decompose on the column. This was evident during the experiment where a silica gel column was done on the DEET with 4-ethyl-3- thiosemicarbazide.
2.8.1. Synthesis of (E)-2-((Diethylamino)(m-tolyl)methylene)-N- ethylhydrazinecarbothioamide with Copper(II)Chloride
.
Observations of the progression of the reaction were recorded. It was noticed that during the reaction mixture turned from brown to yellow in color during refluxing.
Due to air drying, the product of the reaction mixture resulted in a yellow powder-like substance as the product.
Compound B. Copper(II) complex
The tubes of solvent with copper(II) complex were assessed in order to compare the solubility of copper(II) complex in certain solvents.
The tube with the mixture of Copper(II) complex with 1 ml of DMSO was observed; the sonication resulted in a mixture. The same results were observed after the sonication of the copper(II) complex with 1ml of EtOH and copper(II) complex with 1 ml of H2O.
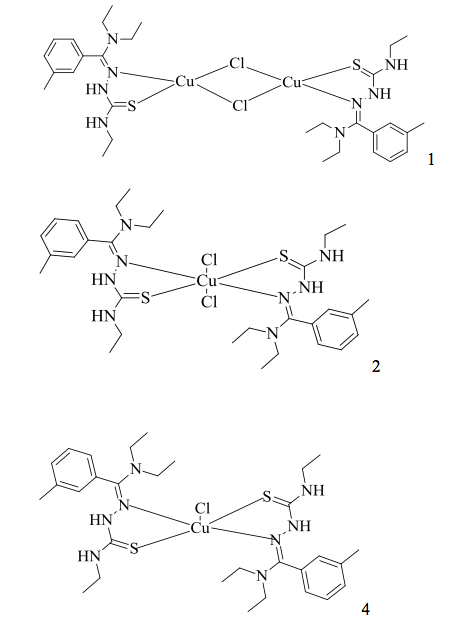
In order to determine the specific product of the synthesis of (E)-2-((Diethylamino)(m- tolyl)methylene)-N-ethylhydrazinecarbothioamide with copper(II)Chloride, we used the procedure and schemes described by Beckford et al.9 as a template. The schemes provided for the copper(II) complex that was produced by Beckford et al.9 allowed us to conclude that our scheme for the production of our copper(II) complex would be very similar.
Scheme 3. Proposed structures of the complexes described by Beckford et al. 9
In comparison to the schemes provided by Beckford et al. 9, the product that we produced simply replaced the phenyl-substituted amino nitrogen of their complex with 4- ethyl-3-thiosemicarbazide. Additionally, the schemes helped us to conclude which of the structures provide by Beckford et al. 9 was most similar to the structure of the copper(II) complex that we produced.
Scheme 3.1. Proposed structures for the synthesis of (E)-2-((Diethylamino)(m- tolyl)methylene)-N-ethylhydrazinecarbothioamide with copper(II)Chloride
Due to the comparison of the FT-IR peaks (shown in Table 1.) presented by Beckford et al. 9 with the peaks of the FT-IR spectrum of our copper(II)complex, we concluded that the product of the synthesis of (E)-2-((Diethylamino)(m-tolyl)methylene)-N- ethylhydrazinecarbothioamide with copper(II)Chloride mostly resembled the structure of (4) which was provided by Beckford et al. 9
3.1. Tables and Graphs
The FT-IR of the ligands and complexes that were produced were observed and compared to similar FT-IR described by Beckford et. al 9 and Lewis et. al. 5 Compound C was analyzed in comparison to the FT-IR of the several Cu(II) complexes of Beckford et al. to decipher which of these complexes most resembled the product of our synthesis. Additionally, FT-IR described by Lewis et al. was used for the analysis of Compound A.
Table 1. IR data for products in cm-1.
|
Assignments |
||
|
Compounds |
A (ligand) |
C (complex) |
|
C=S |
841.16 (m), 1259.94 (m), 1295.86 (w) |
849.27 (w), 1253.09 (m) , 1347.15 (w) |
|
C=N |
1637.87 (w) |
1556.90 (s) |
|
N-H |
3245.10 (b), 3353.44 (b) |
3209.66 (w) |
b= broad, m= medium, s= strong, w= weak
Due to the comparison of the FT-IR peaks presented by Beckford et al. 9 with the peaks of the FT-IR spectrum of our copper(II)complex, we concluded that (E)-2-((Diethylamino)(m- tolyl)methylene)-N-ethylhydrazinecarbothioamide with copper(II)Chloride mostly resembled the structure of (4) which was provided by Beckford et al. 9
Future Work:
Malaria has claimed many lives and has infected numerous people around the world. Though there are treatments, malarial strains are continuing to develop, making it difficult for scientists to provide a truly effective drug to at least suppress the symptoms of malaria. As scientists, we feel an obligation to use our skills and resources to aid in the effort to eradicate malaria. Using the compounds that we have produced, especially (E)-2-((diethylamino) (m- tolyl)methylene)-N-ethylhydrazinecarbothioamide and our copper(II) complex, we hope to establish an effective drug that will inhibit the transmission of malaria and ultimately control the disease.
The compounds that have been described in this study will be tested in biological laboratories in order to assess their effectiveness against malaria-infected cells and the malarial parasite, P. falciparum. Additionally, these compounds will be assessed for usefulness in the potential inhibition of some cancer and Third World diseases such as Chagas disease.
References
[1] Murray, C.; Rosenfield, L.C.; Lim, S.S.; Andrews, K.G.; Foreman, K.J.; Haring, D.; Fullman, N.; Naghavi, M.; Lozano, R.; Lopez, A.D. Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet, 2012, 379, 413-431.
[2] Bjorkman, A.; Bhattarai, A. Public health impact of drug resistant Plasmodium falciparum malaria. Acta Tropica. 2005, 94, 163-169.
[3] cdc. gov Centers for Disease Control and Prevention. Malaria. http://www.cdc.gov/malaria/index.html ( accessed April 26, 2013).
[4] Lobana, T.S.; Sharma, R.; Bawa, G.; Khanna, S. Bonding and structure trends of thiosemicarbazone derivatives of metals-An overview. Coord. Chem. Reviews. 2009, 253, 978.
[5] Lewis, N.A.; Liu, F.; Seymour, L.; Magnusen, A.; Erves, T.R.; Arca, J.F.; Beckford, F.A.; Venkatraman, R.; González-Sarrías, A.; Fronczek, F.R.; VanDerveer, D.G.; Seeram, N.P.; Liu, A.; Jarrett, W.L.; Holder, A .A. Synthesis Characterisation, and Preliminary In Vitro Studies of Vanadium(IV) Complexes with a Schiff Base and Thiosemicarbazones as Mixed Ligands. Eur. J. Inorg. Chem. 2012, 664.
[6] Silva, M.J.; Alves, A.J.; Do Nascimento, S.C. Synthesis and cytotoxic activity of N- substituted thiosemicarbazones of 3-(3,4-methylenedioxy) phenylpropanal. Il Farmaco. 1998, 53, 241-243.
[7] Khayne, S.D.; Smith, G.S.; Lategan, C.; Smith, P.J.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis and in vitro evaluation of gold (I) thiosemicarbazone complexes for antimalarial activity. J. of Inorg. Biochem., 2010, 104, 1079-1083.
[8] Lessa, J.A.; Parrilha, G.L.; Beraldo, H. Gallium complexes as new promising metallodrug candidates. Inorganica Chimica Acta, 2012, 393, 53-63.
[9] Beckford, F.A.; Thessing, J.; Stott, A.; Holder, A. A.; Poluektov O.G.; Li, L.; Seeram, N.P. Anticancer activity and biophysical reactivity of copper complexes of 2-(benzo[d][1,3]dioxol-5- ylmethylene)-N-alkylhydrazinecarbothioamides. Inorg. Chem. Comm., 2012, 15, 225- 229.
[10] Qiu, H.; McCall, J.W.; Won Jun, H. Formulation of topical insect repellent N,N-diethyl-m- toluamide (DEET): vehicle effects on DEET in vitro skin permeation. International Journal of Pharmaceutics, 1998, 163, 167-176.
[11] Carroll, S.P.; Loye, J. PMD, a registered botanical mosquito repellent with DEET-like efficacy. Journal of the American Mosquito Control Association, 2006, 22, 507-514.