Synthesis of Ferrocene
Written by Emily
Introduction – Synthesis of Ferrocene
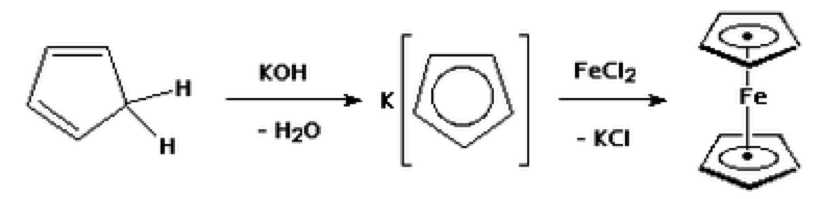
Organometallic sandwich compounds were first synthesized in 1951. Ferrocene was the first organometallic sandwich compound to be made and the structure was not determined until a year after it was first produced. Ferrocene can be treated as two cyclopentadienyl anions liked by Fe2+ with an unusual stability. Cyclopentadiene, which is used to make ferrocene, is very unstable because it undergoes dimerization easily. For this reason, cyclopentadiene must formed just before the reaction to make ferrocene and it should be kept cooled to prevent dimerization from occuring.
Table of Chemicals
Procedure
- In a 100-mL round bottom flask, 8.0 grams of potassium hydroxide, 20 mL of 1,2-dimethoxyethane, and 2 mL of cyclopentadiene were mixed for ten minutes.
- At the end of ten minutes, a double necked glass piece was placed on top and a condenser and separatory funnel were placed in each hole of the double necked piece.
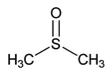
- In a 125-mL Erlenmeyer flask, 2.4 grams of FeCl2 were dissolved in 12 mL DMSO by warming in a water bath for five minutes.
- This FeCl2 solution was then cooled in an ice bath and transferred to the separatory funnel. The solution was added slowly to the flask over a period of ten minutes.
- The flask was then removed and a lid was placed on top. This solution was mixed for five more minutes then poured into a beaker containing 35 mL of 6.0 M HCl and 40 g of ice.
- The flask was rinsed with three 20-mL portions of water to complete the transfer into the beaker.
- The solution was filtered with a Buchner vacuum funnel and the orange ferrocene collected was rinsed with 20 mL of cold water.
- The orange ferrocene was purified via sublimation and the final product was weighed.
Mechanism
Observations
During this procedure, the separatory funnel used had a loose connection and the iron chloride solution was added very quickly instead of drop wise over a ten-minute period. The final product was bright orange and had a mass of 0.308 grams after sublimation.
Percent Yield
Expected yield: 1.0 gram
Final product weight: 0.308 grams
Results
An organometallic sandwich compound, ferrocene, was synthesized by the addition of Fe2+ to two cyclopentadiene rings. The reaction produced bright orange crystals with a final mass of 0.308 grams. The bright orange color was used to verify that ferrocene was successfully synthesized since the melting point was not determined. The percent yield was a low value of 31%. This was due to that fact that some of the iron chloride solution leaked out of the separatory funnel during the experiment.
Questions
- The intermediate, potassium cyclopentadiene, is sensitive to air because it oxidizes easily. To avoid this oxidation, the reaction must be carried out with no air.
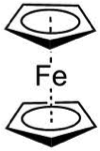
- The structure in Figure 12.1 is the solid phase. The gas phase structure is shown below: