Synthesis and properties of Polyaniline
Abstract
Polyaniline was successfully synthesised using cyclic voltammetry. The redox properties of emeraldine base, a polyaniline derivative, were explored, and it was proved using UV-Vis spectroscopy that emeraldine salt exists in a coiled form when using DMF as a solvent, but in the extended chain form in m– cresol.
Introduction
Inherently conducting polymers (ICPs) are electrically conducting polymers that can by synthesised by the chemical (or electrochemical) oxidation of suitable monomeric materials. One of the most studied ICPs is polyaniline (PAn), as shown in figure 1.
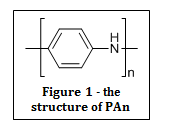
The monomer unit is first oxidised by the formation of a radical cation, which then combine to form dimers and higher oligomers. Each ICP is initially prepared in an oxidised form consisting of a positively charged backbone and anionic species that are incorporated during synthesis to maintain neutrality. By varying the anion that is incorporated, it is possible to alter the physical properties of the polymer chain.
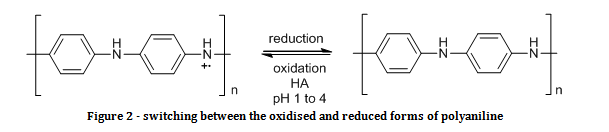
All ICPs can exist in multiple oxidation states. Figure 2 shows how polyaniline can be switched between the oxidised, electrically conducting form, and the reduced, electrically insulating form.
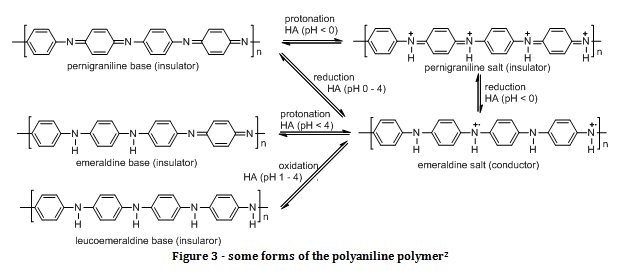
Polyaniline can exist in many forms, with one of its most stable, non conducting forms being called emeraldine base. Figure 3 shows some of the forms of polyaniline. The conductivity of the conducting form of emeraldine base (emeraldine salt) can vary massively depending on the solvent system used (for example, 0.1 S cm-1 in chloroform, and 200 S cm-1 in m-cresol)[i]. This is mainly due to the conformation that the polymer adapts in solution. The polymer can either adapt a compact coil conformation, or it can exist as a straight chain. When the non-conducting polymer is oxidised and an electron is removed, a radical cation species is created. This radical cation is known as a polaron, and enables the polymer to conduct, as shown in the emeraldine salt in figure 3. When the polymer exists as a straight chain then there is sufficient overlap of π orbitals to allow for delocalisation of electrons along the polymer chain. The addition of polarons to the chain gives a driving force for the movement of electrons (to neutralise the positive charge), which explains why emeraldine salt (the only polymer chain with polarons in figure 3) is the only conducting polyaniline.
With respect to the UV-vis spectra of the above compounds, only one band would be expected for the insulating polymers, whereas one would expect three bands in the UV-vis spectra for emeraldine salt. This is because in the absence of polarons there is only one possible transition for an electron when it is excited, which is excitation from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO), in which case the HOMO would be the π bonding valence band, and the LUMO would be the π* antibonding conduction band. On the addition of polarons to the system, there are now three possible transitions for the excitation of electrons. There is still a HOMO to LUMO transition as before, but with the energy of the polaron lying somewhere between the HOMO and LUMO, two other electronic transitions become possible. Transitions can now occur from the HOMO to the polaron and from the polaron to the LUMO as demonstrated in figure 4. Unless the energy of the polaronlies exactly half way between the HOMO and LUMO, then each transition will have a different energy, and will each absorb a different wavelength of light in order to undergo the transition, as such, three bands should appear in the UV-Vis spectrum of the conducting emeraldine salt polymer.
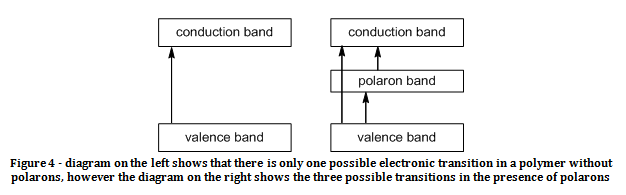
Figure 4 – diagram on the left shows that there is only one possible electronic transition in a polymer without polarons, however the diagram on the right shows the three possible transitions in the presence of polarons
The aims of this experiment were to prepare polyaniline by potentiodynamic polymerisation using cyclic voltammetry, and to study the redox behaviour and acid/base chemistry of polyaniline using a commercially available sample of emeraldine base.
Experimental
Preparation of the stock polyaniline solution
Aniline (4 g) and concentrated hydrochloric acid (20 ml) were added to water (200 ml) and stirred for ten minutes.
Potentiodynamic synthesis of polyaniline
Using the stock solution (10 ml), polyaniline was grown onto an ITO coted glass electrode using cyclic voltammetry using the following parameters:
– Initial potential = final potential = -0.2 V
– Lower potential limit = -0.2 V
– Upper potential limit = 0.7 V
– Number of growth cycles = 10
– Scan rate = 100 mV s-1
The ITO plate changed from being clear to blue, to green, to yellow, and repeated this cycle until the experiment was stopped.
Chemical and spectroscopic properties of emeraldine base
Dissolve emeraldine base (EB) (0.5 mg) in dimethylformamide (DMF) (20 ml). The blue solution was divided roughly into five portions and the following tests were performed.
a) The UV-Vis spectrum of the emeraldine base was recorded in a 1 cm glass cuvette, using DMF as a reference solvent (appendix 2). The value was λmax was 614 nm.
b) To this solution was added camphosulphonic acid (HCSA) (10 mg), and the UV-Vis spectrum of the resulting green solution was recorded (appendix 3).
c) To another portion of the original EB solution was added hydrazine (5 drops). The UV –Vis spectrum of the resulting solution was recorded after the mixture had been left to react for ten minutes. There was very little visible colour change (appendix 4). The value of λmax was 626 nm.
d) Another portion of the original EB solution was added to solid ammonium persulfate (0.1 g) and was shook vigorously for five minutes. The UV-Vis spectrum of the resulting grey solution was recorded (appendix 5) the value of λmax was 519 nm. The mixture was left to react for a further five minutes, after which the UV-Vis spectrum of the now clear solution was recorded (appendix 6). The value of λmax was 546.5 nm.
e) The UV-Vis spectrum of a sample of EB in m-cresol that had been treated with HCSA (appendix 7).
Results and Discussion
Hydrazine is a reducing agent, so the species formed is leucoemeraldine base in part (c) of the experiment. In part (d) of the experiment, the ammonium persulfate used was an oxidising agent, meaning that according to figure 3 pernigraniline base was formed.
The additions of HCSA protonated the emeraldine base, causing it to be become the protonated, electrically conducting material, emeraldine salt.
The UV spectrum of the emeraldine salt in DMF only gave one peak. This was indicative that polymer existed in the coiled form in this solvent. This disrupts the conjugation and the overlap of p orbitals and polaron energies, as such; there is only one possible electronic transition possible.
The provided UV spectrum of emeraldine salt in m-cresol gave three absorption bands, showing that in this solvent the emeraldine salt existed in the extended form. There is a strong absorption below 300 nm, which probably corresponds to the π to π* transition, as that would be the transition with the highest energy (and energy is inversely proportional to wavelength). There was another absorption at around 390 nm due to the polaron to π* transition. There was a very broad absorption from 800 nm above. This is due to the π to polaron transition. The absorption was broad because there are many possible transitions to the polaron energy band, as demonstrated in figure 5.
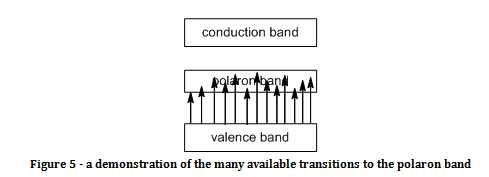
The fact that the absorption for the π to polaron band was at a higher wavelength indicates that the polaron band is closer to the valence band than the conduction band (since energy is inversely proportional to wavelength).
During the electrochemical polymerisation of polyaniline, there were several colour changes. This was due to the fact that the polymer was being oxidised and reduced in a cycle between leucoemeraldine, emeraldine and pernigraniline. The cyclic voltammogram of this process is shown in appendix 8.
[1] Younan Xis, Joanna M. Wiesinger, and Alan G. MacDiarmid, Chem. Matter., 7, 1995, 443 – 445
[1] Peter Innis, CHEM301 lecture course, University of Wollongong, 2011
