Synthesis of Ruthenium(II) and Molybdenum(V) using dimethylsulfoxide (DMSO) as a ligand
Synthesis of Ruthenium(II) and Molybdenum(V) using dimethylsulfoxide (DMSO) as a ligand
By: Cooper Semple and Nathaniel Hurd
- Abstract– In this experiment two compounds tetrakis(dimethylsulfoxide)dichlororuthenium(II) and dichlorodioxobis(dimethylsulfoxide)molybdenum(VI) were synthesized using dimethylsulfoxide as the ligands. This was performed by adding DMSO to RuCl3(H2O)3 as well as to MoO3 then by heating and finally vacuum filtration to collect our final products. We concluded that both compounds were made correctly with few impurities as well as how each metal was bonded to DMSO. This work was done by taking a melting point of each compound and then comparing that to the theoretical melting points as well as taking IR spectras of each compound and then figuring out how the DMSO peak had been shifted.
- Introduction– In this experiment we synthesized two metal compounds, Ruthenium(III) and Molybdenum(VI) with dimethylsulfoxide. The main purpose of this lab was to see which of the metals would bind to the ligand dimethylsulfoxide either through the sulfur or through the oxygen. We synthesized the two products RuCl2(DMSO)4 and MoO2Cl2(DMSO)2. By taking the IR spectra we can then determine how these metals are bonded to DMSO. We also took the melting points of both compounds as well as the crude MoO2Cl2(DMSO)2 to judge the purity of each compound.
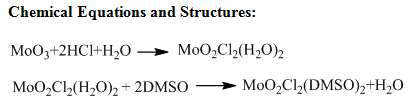
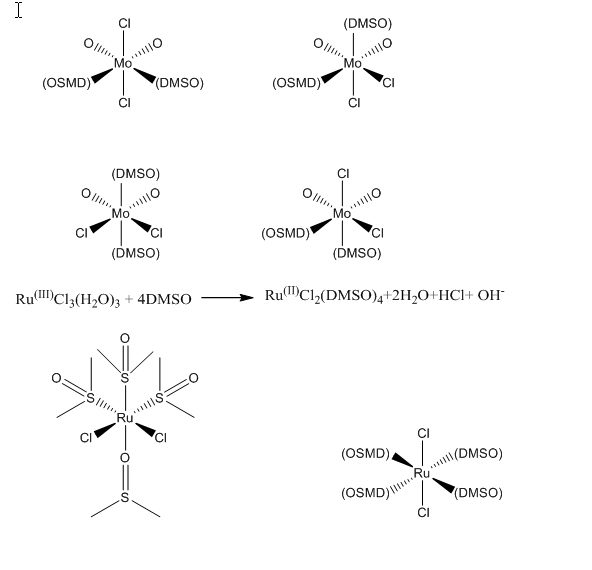
- Materials and Methods
Reagents: Ruthenium(III) chlodie hydrate: Aldrich
Molybdenum(VI) trioxide: J.T. Baker Chemical company
HCl: EM Science 36.5%
Ethyl Ether Anhydrous: Fisher Chemical
Dimethylsulfoxide: Fisher Chemical
Procedure:
Synthesis of RuCl2(DMSO)4: To synthesize the RuCl2(DMSO)4 we followed the procedure previously described.
Synthesis of MoO2Cl2(DMSO)2: To synthesize the MoO2Cl2(DMSO)2 we followed the procedure previously described with one difference. In the recrystallization of MoO2Cl2(DMSO)2 we used 0.5 grams of the crude product as well as 5mL of acetonitrile. We then rinsed this with 2.5mL of acetonitrile. This is because we used half of what the procedure asked for.
- Data and Calculations– To determine the percent yield of both MoO2Cl2(DMSO)4 in its recrystallized form as well as the crude form and RuCl2(DMSO)4 we took the amount of our starting metals as the limiting reagents to get a theoretical yield. This was then compared to the final weights of our final products to get a percent yield.
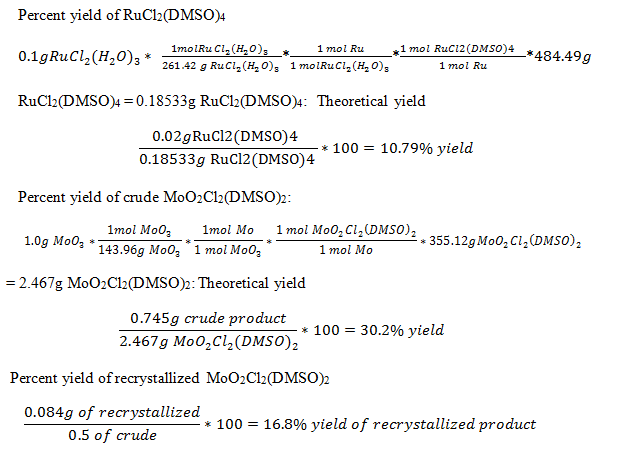
-Some product was lost in the transfer before recrystallization so the theoretical yield is less than the final weight of the crude. These percent yields for all compounds are still pretty low. This could be because of crude product being lost before recrystallization as well as the crude product being impure.
- Discussion: In this lab we synthesized the two products Dichlorodioxobis(dimethylsulfoxide)molybdenum(VI) and Tetrakis(dimethylsulfoxide)dichlororuthenium(II). The main purposes of this lab was to see if we could synthesize both products as well as to see if the DMSO would bind to each metal by either the oxygen or the sulfur atoms. We figured this information out by taking both the melting point as well as IR spectra. By taking the melting points of RuCl2(DMSO)4 and MoO2Cl2(DMSO)2 as well as the crude MoO2Cl2(DMSO)2 and comparing them to the theoretical yields we found that all we pretty close to the theoretical yields but there still might have been some impurities left in each product. When looking at the theoretical yield for RuCl2(DMSO)4 the theoretical yield should be somewhere around 192-198. When looking at our actual melting point we got a melting point of around 194.5-200.1. This was pretty spot on to the theoretical yield, but it was possible that there were some impurities or we were a little slow at reading the thermometer. When looking at both the crude and recrystallized melting points for MoO2Cl2(DMSO)2 they should be pretty close theoretical wise. We found that the theoretical melting point for MoO2Cl2(DMSO)2 should be somewhere around 172-173. When taking our crude products melting point we found that it melted from 154.9-168.5. This had a very broad range of melting points, so we can be pretty sure that the crude product still had impurities. As for the recrystallized product, we got a melting point of 176.4-178.2. This was a little over the theoretical melting point but since our crude product’s melting point was below these numbers I think we made an error at reading the thermometer.
After the melting points were recorded, the IR spectra was taken for each product we made. We compared these spectra to the IR spectra of dimethylsulfoxide. The main peak to look at while looking at free DMSO, is at 1050cm-1. When looking at RuCl2(DMSO)4 we found that the peak had been upshifted to 1093cm-1. This shows that the Ru will be bonded through the sulfur on the DMSO. This also shows that the sulfur-oxygen bond will be shortened. As for the crude and recrystallized MoO2Cl2(DMSO)2 the peak was downshifted to 1033cm-1 for the crude product and 1034cm-1 for the recrystallized product. This shows that both of these compounds the Mo will be bonded to the DMSO through the oxygen. Another way to look at this would be by looking at hard-soft acid-base theory. In this experiment the main thing we are looking at would be the oxidation states of the metal compounds. Since MoO2Cl2(DMSO)2 has an oxidation state of six and RuCl2(DMSO)4 has an oxidation state of two, MoO2Cl2(DMSO)2 will be a harder acid than RuCl2(DMSO)4. When binding, the harder acid will bind more strongly to the harder base which is the oxygen atom, and the softer acid will bind more strongly to the softer acid which is your sulfur.
- Questions:
1.) I would expect that since both platinum and mercury are relatively large atoms that both of these would bind with the sulfur on the DMSO. This is also because the sulfur ion will be larger than the oxygen ion. Just the opposite would happen with the zinc and iron, they would both bind to oxygen because both of these are smaller in size as well as the oxygen atom. I am not including how charge might come into play because this might have an effect.
2.) Hard-soft acid-base theory goes over how typically soft acids and soft bases will form stronger bonds then say a soft acid and hard base. In this lab the biggest criteria we are looking at would be the charge on the metals. In this case the Molybdenum has an oxidation state of six. This makes it a harder acid then Ruthenium which has an oxidation state of two by the time it is bonded with the dimethylsulfoxide. As for the two bases, oxygen and sulfur, oxygen will be a harder acid than sulfur and therefore bind the Molybdenum.
3.) The main purpose of recrystallizing the MoO2Cl2(DMSO)2 is to increase the purity of the product. This can be shown by the melting point ranges of the crude product and the recrystallized product. The crude product had a much more broad range of temperatures verses the recrystallized product as well as the recrystallized product having a much closer temperature range to the theoretical melting point.
4.) I would think that the correct structure for all the possible isomers of MoO2Cl2(DMSO)2 would have the two DMSO ligands in the trans. This is because of sterics.
- Conclusion: In this experiment our main objective was to synthesize the two compounds RuCl2(DMSO)4 and MoO2Cl2(DMSO)2. Once these compounds were created we proved that these compounds were the actual compounds by taking the melting points. One step further we also figured out how the dimethylsulfoxide was bonded to both of the metals. By taking IR spectras as well as using our knowledge on hard-soft acid-base theory we found that the Ruthenium compound was bonded to DMSO by the sulfur ion while the Molybdenum compound was bonded to DMSO by the oxygen ion.
References: Szafran, Z.; Pike, R.M.; Singh, M.M. Mircroscale Inorganic Chemistry. John Wiley and Sons: New York, 1991, pages 218-222. (b) Inorg. Syth. 1997, 31, 246-247
Inorganic Synthesis, Volume 3. Edited by Allen H. Cowley 1997 Inorganic Synthesis, Inc.
MSDS of Tetrakis(dimethylsulfoxide)dichlororuthenium(II)
