Esterification of Benzocaine
Esterification of Benzocaine
By: Nick Makowski and Monica Schaeffer
Purpose:
To practice the techniques of refluxing and vacuum filtration to perform an esterification reaction to create benzocaine from p-aminobenzoic acid
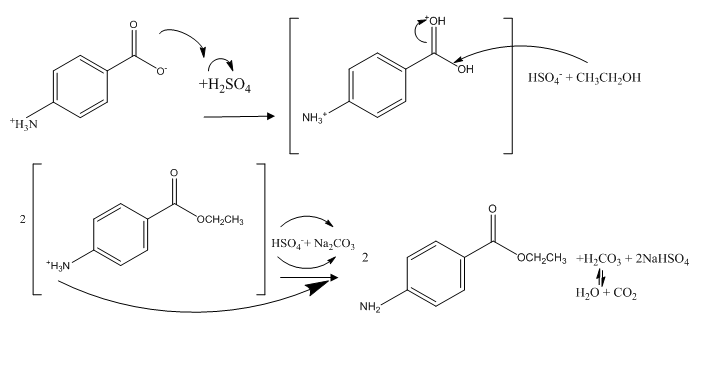
Chemical Structures/Reactions/Mechanisms:
Results:
Physical Appearances-
After ethanol and sulfuric acid added to p-aminobenzoic acid, solution became clumpy and then turned into a white foam
After refluxing, solution became more solid
Upon filtration, white benzocaine crystals formed
Mass of Substances-
p-aminobenzoic acid: 1.201g
benzocaine: 0.368g
Na2CO3: 11mL
Melting Point-
Known= 88-90 °C
Experimental= 89-90 °C
TLC/Rf-
Solvent front- 5.8 cm
Solvent- 1:1 hexane/diethyl acetate
p-aminobenzoic acid Rf value: 0.19
benzocaine Rf value: 0.34
IR Spectra-
See attached graphs
Calculations
Rf values:
Solution movement/Solvent movement= Rf value
Ex: 1.1cm /5.8cm= .19
Discussion
A) Questions
1) Because the product had a different Rf value than the starting product, the benzocaine is obviously not still p-aminobenzoic acid. Also, as the dots were fairly distinct, it seems to lead to the conclusion that there is no evidence that there is presence of the starting material in the reaction product.
2) The experimentally determined melting point tells us that our product is very pure. It was within the narrow range of the known melting point for our product, which indicates purity of our substance.
3) Our melting point range was almost exactly the same as the known melting point range for this compound. The known range is 88-90 °C, while our experimental melting point was 89-90 °C.
4) While the range from 450-1700 looks fairly similar for both the p-aminobenzoic acid and the benzocaine, there are some notable differences. These include the absence of the bump at 3660 on benzocaine’s spectrum that is there on the starting material’s spectrum. Also, there is a strong peak between 460 and 500 on benzocaine’s, and only a strong peak at 460 for the starting material’s spectrum. There is a moderate peak for p-aminobenzoic acid at 2982.3, while benzocaine’s peak at the exact same spot is not as strong. These observations could lead to the conclusion that benzocaine is no longer p-aminobenzoic acid.
B) Conclusion
It seems we were successful in producing benzocaine by esterification based on results of melting point, IR spectroscopy, and TLC analysis. The melting point we received was within the range of the known melting point. Also, the TLC analysis produced two distinct, different-valued points that were obviously different substances. IR spectroscopy showed similar, but distinct products. We did not have any problems during this experiment. These results indicated that we had a pure product.