Simple and Fractional Distillation Experiment
Distillation
By: Tim Dimond
Introduction/Purpose
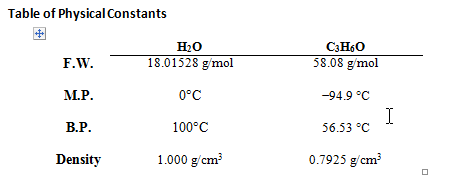
The purpose of this lab was to purify an unknown mixture of acetone/water by both fractional and simple distillation. This process is a viable method for separation of these two liquids because of the large separation in their boiling points.
Material
-Water and acetone mixture
Equipment
– A setup for fractional distillation and simple distillation
-100 mL round bottom flask
-Volume measuring equipment
-Heating equipment
Procedure
Obtain 70 mL of the acetone/water solution in a round bottom flask with several boiling chips. Perform a fractional distillation, recording the temperature with every 5 mL of distillate. Record the weight of the acetone, water, and remaining fractions. Do the same procedure again, but with a simple distillation set up without a column condenser.
Results and Observations
Boiling point of acetone component = ~60 OC based on results
Boiling point of water component = ~ 100 OC based on results
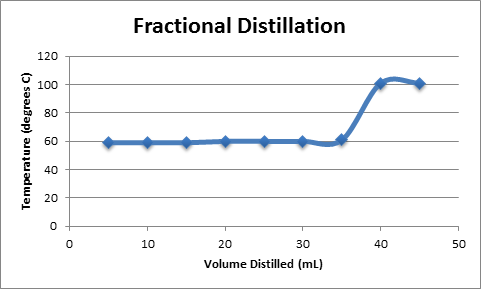
Fractional Distillation
Mixture Weight= 64.68 g
Fraction 1 – 26.17 g, 36 mL
Fraction 2 – 10.01 g, 10.5 mL
Leftover in round bottom flask – 19.98g, 20 mL
DensityFD= 0.727 g/mL
%Acetone= 127%
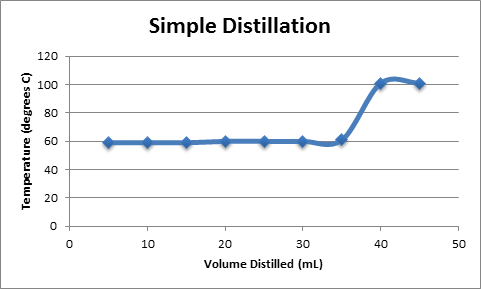
Simple Distillation
Mixture Weight= 64.98 g
Fraction 1 – 28.04 g, 35 mL
Fraction 2 – 12.45 g, 13 mL
Leftover in round bottom flask – 22.98 g, 24 mL
DensitySD= 0.801 g/mL
%Acetone= 92%
A plot of Temperature vs. Volume of Distillate is shown in Figure 2. The plot shows a function with a slope of zero, corresponding to the removal of acetone from the solution, followed by a sharp increase in temperature as water begins to boil off from the solution. Both fractional and simple distillation plots are shown, although they are nearly identical.
Questions
- Answered above
- Answered above
- Answered above
- The fact that our results say the factional distillation yielded 127% acetone is impossible, but may prove that the fractional distillation is more effective. Further experiment would be necessary, because the 127% value is clearly based on some sort of measuring error.
- Industry frequently uses distillation for both batch an continuous processes. One example if distillation in petroleum refineries.
- The boiling point of a liquid is the temperature at which the vapor pressure of that liquid exceeds the vapor pressure of the air surrounding it. When this happens, the liquid boils and a phase change occurs, as the liquid becomes a gas.
- Heating a closed system can cause a build up of pressure and energy, which may eventually result in an explosion or dangerously fast release of this built up energy.
References
– Lab handout
– Wikipedia