Sodium Borohydride Reduction of 2-methylcylohexanone
Purpose
The object of this experiment was to reduce the ketone group of a cycloalkane into an alcohol group. This reduction forms cis and trans isomers and the experiment intended to determine which was more favorable.
Analysis
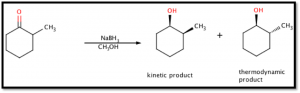
The object of this experiment was to reduce the ketone group of 2-methylcyclohexanone to form 2-methylcyclohexanol (see figure 1 below). Infrared spectroscopy, an important analytical technique, was introduced to examine the purity of the product. Infrared spectroscopy analyzes a chemical’s transmittance over a wavelength spectrum and allows for identification of certain functional groups and characteristics. This reduction reaction yields two possible isomers and the experiment intended to determine (via gas chromatography) whether the thermodynamic or kinetic product was more favorable (see Figure 1 below).
Figure 1: Net Reaction
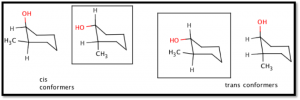
The two isomeric cyclohexanols can exist in four chair conformations (see Figure 2). The cis isomer’s most stable conformer is with the hydroxyl group in the equatorial position and methyl group in the axial position, while the trans isomer is most stable in the diequatorial conformer. The hydroxyl group is more sterically encumbering than the methyl group and is more stable in the equatorial position. Both of these cis and trans conformers are in the ‘gausche’ position, and are therefore high-energy molecules (see Table A).
| Chemical | Energy (kJ/mol) |
| methylcyclohexanone |
63.43 |
| cis-2-methylcyclohexanol |
66.36 |
| trans-2-methylcyclohexanol |
59.91 |
Table A: Relative Energy Comparison of Most Stable Conformers
Trans-2-methylcyclohexanol was identified as the thermodynamic product because it is more stable and lower in energy than the cis-isomer (see Table A above). The thermodynamic product has a higher activation energy, which was expected to affect the yield of each isomer. The cis-isomer was assumed to be the kinetic product, considering its higher relative energy, which is even greater than the reactant.
Sodium Borohydride Reduction
The procedure to reduce 2-methylcyclohexanol was relatively simple. Cyclohexanone (1.2g) was cooled and added to sodium borohydride (200mg). A vigorous, bubbling reaction took place for several minutes. After the reaction ceased, sodium hydroxide (5mL) was added to the solution to decompose the borate ester, leaving a cloudy solution. Water (4mL) was added, allowing the product to separate as a mostly clear upper layer. The layer was removed and then two successive methylene chloride (2mL each) additions were used to extract the remaining product (in the bottom layer). The recovered products were dried and the methylene chloride was subsequently boiled away. During the boiling, methylene chloride vigorously formed bubbles and carried some product away. In further experiments, temperature must be more carefully moderated, or a larger flask must be used. IR spectrum and gas chromatography were conducted with the product.
| Theoretical Yield | Actual Yield | Percent Error |
| 1.314 mL | 0.65 mL |
49.50% |
Table B: Product Yield
The IR spectrum indicated that the product was purely an alcohol, showing a large valley centered around wavenumber 3400, and revealed no structure around wavenumber 1720, where the ketone group was found in the reactant IR (see Table C).
| Group (assumed) | Wavenumber (cm-1) |
| Ketone (reactant IR) |
1720 |
| Alcohol (product IR) |
3400 |
| Carbon Fingerprints | 1500-600 |
Table C: IR Spectrum Anomalies
Gas chromatography further confirmed the purity of the product, showing the two expected isomers. Comparing retention time to established boiling points allowed for identification of each isomer. The thermodynamic (trans) product was highly favorable, observed as 85% of the product (see Table D below). The experimental yielded about 50% of the expected product (see Table B).
| Chemical | Peak Area | % Yield of Isomer | Retention Time (s) |
| methylcyclohexanone | .305 cm2 |
4.4 |
|
| cis-2-methylcyclohexanol | 0.385 cm2 |
15.5 |
30.6 |
| trans-2-methylcyclohexanol | 2.1 cm2 |
84.5 |
32.7 |
Table D: Gas Chromatography Data
In conclusion, the experiment was highly successful in reducing the ketone group of 2-methylcyclohexanone to an alcohol group using sodium borohydride. . It was determined that in this scenario, the thermodynamic product is generously favored. The procedure was simple and repeatable; refinements should improve the percent yield. This was a valuable experiment that utilized important analytical tools like the IR scan and gas chromatography.
Sample Calculations
Starting Material
(mL/0.9240 g) * 1.2 g = 1.3 mL 2-methylcyclohexanone
Theoretical Yield
1.20 g ketone * (mol ketone/112.17g) * (mol alcohol/mol ketone) * (114.19g/mol alcohol) * (mL/0.930 g) = 1.31 mL 2-methylcyclohexanol
Percent Yield
(Actual yield/theoretical yield) * 100 = % yield
(.65 mL/1.31 mL) * 100 = 49.5%
Peak Area
Peak Area = Peak Height * Width (@half height)
(cis product) 3.85 cm * 0.1 cm = 0.385 cm2
Retention Time
Retention Time = (dxsolvent-dxair)/ds
(cis product) (4.4 mm – 0)/ (1mm/s) = 4.4 seconds