Preparation of 1-Bromobutane and 2-Chloro-2-Methylbutane Using Sn2 and Sn1 Mechanisms
Written by Katrina
Abstract: Haloalkanes are being used every day in the industry: as household solvents reagents, anesthetics, freons, and pesticides to name a few. These haloalkanes can be produced through nucleophilic substitution reactions also known as SN2 and SN1 reactions and it was through this that the haloalkanes 1-bromobutane and 2-chloro-2methylbutane were produced. An SN2 reaction was first carried out to synthesize 1-bromobutane from 1-butanol with the help of hydrobromic acid. The obtained percent yield of the product 1-bromobutane was 61% with a measured boiling point of 96°C; a boiling point lower than the its actual one of 99°C indicating that the 1-bromobutane obtained might not be completely pure. However, an SN1 reaction was used to produce 2-chloro-2-methylbutane by reacting 2-methyl-2-butanol with hydrochloric acid. 2-chloro-2-methylbutane resulted with an 82% yield and boiling point of 83°C which was lower than the actual boiling point of 85°C also indicating impurities.
Introduction
Alkyl halides also known as haloalkanes have an important role in every day usage. Some common uses of haloalkanes are found in solvents, reagents, anesthetics, freons and pesticides. Haloalkanes such as methylene chloride(CH2Cl2) and chloroform(CHCl3) are primarily being used in the industry and as household solvents. They are also used for dry-cleaning and removing spots.Although these are being used in households they should be used with precaution because they can possibly be toxic or even carcinogenic. Alkyl halides also help in the production of making complex molecules such as organometallic reagents, which is used for organic synthesis.Some alkyl halides such as chloroform and halothane are types of anesthetics that are used for minor procedures in surgeries. However, some alkyl halides are also a danger to the community. Freons also known as chlorofluorocarbons, or CFCs are a type of fluorinated haloalkane that substitutes ammonia as a refrigerant gas. If Freon-11, another type of haloalkane, is released into the atmosphere it will react with the earths protective ozone layer. As they diffuse to the stratosphere the chlorine atoms will catalyze the decomposition of ozone into oxygen. An example of a toxic alkyl halide is DDT (DichloroDiphenylTrichloroethane); an ounce of DDT will kill a person but will protect an acre of land from insects.1
Because haloalkanes are commonly used everyday it is important to look at how these products are being produced through aliphatic substitution mechanisms.
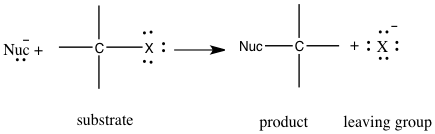
The use of nucleophilic substitution is being studied in the unimolecular reaction, SN1, and bimolecular reaction, SN2. 2 Nucleophilic substitution is a type of reaction where an electron nucleophile bonds or attracts a positive or partially positive charge that is on an atom or set of atoms known as the leaving group; aside from the leaving group, the positive or partially positive atom is called an electrophile.2 In figure 1, we can see how nucleophilic substitution occurs. A nucleophile, which is represented by Nuc, attacks the central carbon, which is also attached to the leaving group represented by an X, which will also leave to form its new bond with its lone pairs.
The two types of mechanisms that are used in this experiment are SN1 and SN2 mechanisms in which S stands for chemical substitution, N stands for nucleophile and the number is the type of rate determining step.2 For this experiment, the SN2 mechanism will focus on the synthesis of 1-bromobutane from 1-butanol. The SN1 mechanism will allow for the synthesis of 2-chloro-2-methylbutane from 2-methyl-2-propanol.
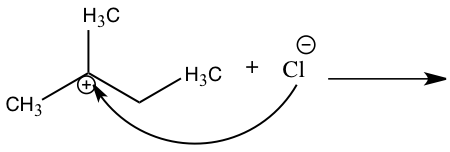
An SN2 reaction is classified by looking at the electrophile’s carbon structure. In an SN2 reaction the central carbon can be primary or secondary. Knowing if the nucleophile is either strong or weak can also help distinguish what mechanism is being used. A polar aprotic solvent is usually used by a SN2 mechanism. 2 The SN2 mechanism is also represented by a direct attack of the nucleophile in one step because bonds are breaking and forming at the same time forcing the leaving group to leave.3 In Figure 2, we can see how the nucleophile attacks the electrophile, causing the leaving group to leave.
Figure 2: SN2 Mechanism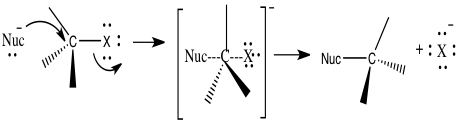 |
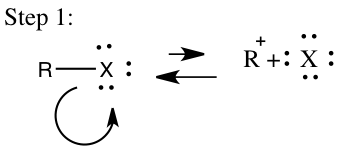
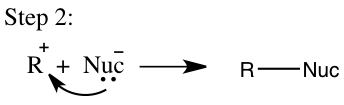
In addition to the SN2 mechanism an SN1 mechanism will occur and in order for it to occur it has certain requirements that need to be reached. An SN1 mechanism is classified as a multistep process in which a strong nucleophile isn’t present and a tertiary carbon is favored.2 The SN1 will usually involve a polar protic solvent and not an aprotic solvent. This reaction will occur in two steps in the first step the leaving group leaves to form a carbocation, which will then be called the rate-determining step and is seen in step 1 on Figure 3. In the second step shown in Figure 3, the carbocation will then obtain a nucleophile and the reaction rate will depend on the concentration of the alkyl halide, which makes it a first order mechanism.4
Overall, we can see how these mechanisms are being used to acquire the final product that is desired. Although we acquired these products, there are also side reactions that occur such as Elimination 1(E1) and Elimination 2(E2).5 Elimination will occur when there is a loss of two atoms or groups from the substrate and are accompanied by substitution reactions.2 An E1 reaction is similar to an SN1 reaction because it requires the ionization of a carbocation and prefers a tertiary carbon rather than a primary carbon. When an E1 reaction occurs the carbocation intermediate will react in different ways, which will give a mixture of products. The E2 reaction also corresponds a SN2 reaction because this elimination reaction only occurs when a strong base is present. The E2 reaction follows Zaitsev’s rule that will predict which possible products will be the major product.2
With the use of SN1 and SN2 reactions, their mechanism will allow the yield of haloalkanes and their side products. The purpose of this experiment is to produce 1-bromobutane through a SN2 reaction and 2-chloro-2-methylbutane through a SN1 reaction. These products or haloalkanes are important because they are commonly used everyday in households and in hospitals.
Results
Table 1: Product Results
| 1-bromobutane | 2-chloro-2-methylbutane | |
| Boiling point (°C): | 96 | 83 |
| Product mass (g.): | 8.478 | 22.064 |
| Percent Yield (%): | 61 | 82 |
With the use of the SN2 mechanism, 61% of 1-bromobutane was recovered. With the product recovered, a boiling point of 96°C was observed. In addition to the SN2 mechanism, an SN1 mechanism was used and recovered 82% of the product, 1-chloro-2-methylbutane with a boiling point occurring at 83°C.
Discussion
Alkyl halides also known as haloalkanes play an important role in industrial uses. Haloalkanes can be found in households in cleaning solutions and spot removers. They are also found in pesticides that kill insects such as mosquitos, fleas, and lice.2
With the uses of haloalkanes we can see how important it is in our daily life. The different types of haloalkanes are grouped in different classes such as alkyl halides, vinyl halides, and aryl halides.The alkyl halide chloroform is used as a solvent and 1,1,1-trichloroethane is used as cleaning fluids that are found in homes. Tetrafluoroethylene is an example of a vinyl halide that is used in Teflon, which is found in cookware. Although some haloalkanes are beneficial some haloalkanes such as thyroxine is not. Thyroxine is an aryl halide that is also known as the thyroid hormone. 2
The product 1-bromobutane is found to cause hepatotoxicity, reduction of glutathione (GSH), and increase serum activities in female mice.6 Another role of haloalkanes is found in the interaction with aluminum and 2-chloro-2-methybutane. With the interaction between aluminum and 2-chloro-2-methylbutane it will lead to the formation of different ions such as AlCl4.7 Because of the importance of haloalkanes it is important to understand the reactions in which they occur.
In order to successfully produce the products 1-bromobutane and 2-chloro-2methylbutane the mechanisms of SN2 and SN1 were used.
Figure 4: Mechanism for reaction of 1-butanol to 1-bromobutane
Step 1: Pronation of leaving group (OH)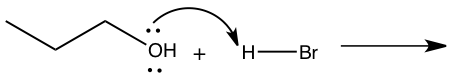 Step 2: Attack of Carbon by nucleophile Step 2: Attack of Carbon by nucleophile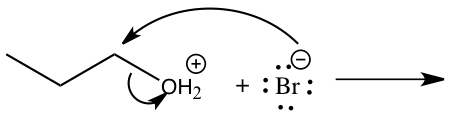 Step 3: Production of 1-bromobutane Step 3: Production of 1-bromobutane
|
In the SN2 reaction, 1-bromobutane was produced through techniques such as simple distillation and extraction(refer to experimental). By looking at Figure 4, we can see how the synthesis of 1-bromobutane from 1-butanol using the SN2 mechanism works. Because the OH group in 1-butanol is not a good leaving group the reaction was conducted in hydrobromic acid which makes it an SN2 mechanism.5 The first step is to pronate the alcohol that will then give an oxonium ion and with the oxonium ion it will do a displacement from the bromide ion to form bromoalkane and water. In this reaction, the water will become the leaving group and the bromide ion will become the nucleophile. The products needed to form in this mechanism are 1-bromobutane and water.5
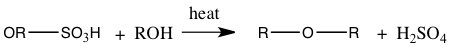
With the distillate obtained from simple distillation and extraction of the distillate the final product of 1-bromobutane boiled at 96°C(as seen in Table 1) which is 3° lower than the actual boiling point of 99°C. The boiling point of 96°C measured meant that the 1-bromobutane attained was not pure. Substances are impure because there may be a presence of another substance. The percent yield of 1-bromobutane was 61.5%. This percent yield means that we didn’t acquire 100% of its products. Because of a low percent yield, this may because during this experiment an elimination reaction instead of an SN2 reaction may have occurred. A bimolecular elimination reaction, E2, will compete with an SN2 process.3 An E2 reaction will occur if a strong base is present.2 Also, in this experiment side products were also formed. The amount of side products like our aqueous solution being taken out may be very high. One side product could have formed by sulfuric acid reacting to the alcohol, forming alkyl hydrogen sulfate and when heated it could have reacted with alcohol to create the side product dialkyl ether.5 The side products that may have produced can be seen in Equation 1.
Equation 1: Side Products of 1-bromobutane
Alkyl hydrogen sulfate production: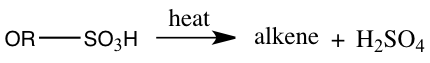
Ether production: |
With these side products consuming alcohol, it will then give a low yield of the bromoalkane, 1-bromobutane.
Figure 5: Mechanism for reaction of 2-methyl-2-propanol to 2-chloro-2methylbutane
Step 1: Pronation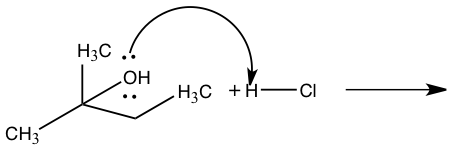 Step 2: Carbocation formation Step 2: Carbocation formation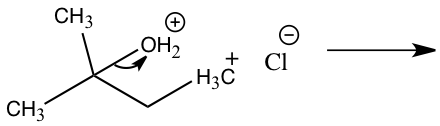 Step 3: Nucleophile attack Step 3: Nucleophile attack
|
The other product produced during an SN1 reaction is 2-chloro-2-methylbutane from 2-methly-2-propanol. In Figure 5, 2-methyl-2-propanol and hydrochloric acid produced 2-chloro-2-methylbutane through an SN1 reaction. In this reaction the leaving group will be water from 2-methyl-2-propanol and the nucleophile is chloride. This mechanism will have three steps in which the first step will be the pronation of the alcohol by hydrochloric acid to create a better leaving group. The second step will be the slowest which is the rate-determining step where the carbocation is formed by removing the alcohol. The third step is the fast step in which the nucleophile attacks the carbocation which will then water and 1-chloro-2-methylbutane.5
With the use of simple distillation and extraction the product, 2-chloro-2-methylbutane reached a boiling point of 83°C which is 2°C lower than its actual boiling point of 85°C. In addition to the last product formed, 2-chloro-2-methybutane also had impurities present because the boiling point was lower than what it should have been. These impurities could have been cause by side reactions such as an E1 reaction. The E1 elimination results from the loss of a proton from the carbocation and a side product of 2-methylpropene may have formed. In 2-chloro-2-methylbutane the total product acquired was 19.1 grams. With that the percent yield was calculated and was 83%. Although the percent yield is high, it didn’t reach the percent yield of 100% because the side product of 2-methylpropene will form when 2-methyl-2-propanol carbocation goes through an elimination reaction.3 Another side product formed is tert-butyl ethyl ether, which will occur during the SN1 reaction. The side products being formed will cause a low percent yield and may also be the reason of 2-chloro-2-methylbutane being impure.
Conclusion
With the reactions carried out, the desired haloalkane products of 1-bromobutane and 2-chloro-2-methylbutane were obtained. The SN2 reaction allowed 1-butanol to be synthesized to 1-bromobutane. In this process we obtained a percent yield of 61%. To determine if the product 1-bromobutane was pure its boiling point was taken and it reached 96°C. Because this boiling point reached a temperature that is a couple of degrees lower than its actual boiling point, we can assume that the synthesized substance contained impurities. In the second mechanism used, we used an SN1 reaction to produce 2-chloro-2-methylbutane from 2-methyl-2-propanol. The amount of 2-chloro-2-methylbutane obtained was 82% and also reached a boiling point of 83°C. Like the product synthesized from the SN2 mechanism, the product in the SN1 mechanism also had a lower boiling point temperature compared to its actual boiling point meaning it may not be pure 2-chloro-2methylbutane. Overall, the use of the mechanisms SN2 and SN1, formed the products of 1-bromobutane and 2-chloro-2-methylbutane. Because low yields were obtained as well as boiling points, this experiment needs to be modified so better yields of the products can be obtained. Perhaps, additional research could be done to see if the haloalkanes produced have conditions that will decrease or eliminate the production of side products.
Experimental
1-Bromobutane Formed by SN2 Reaction
To a round bottom flask(100mL) 0.10mol of sodium bromide(10.3grams), water(12mL), and 0.10mol of 1-butanol(9.1mL) was added. The flask was then mixed thoroughly and submerged into an ice bath. Concentrated sulfuric acid (12mL) was added in the flask while it is swirling and being continuously cooled. Boiling chips were added to the flask in order to prepare it for heating under reflux. The assembly of a reflux condenser and other components is then attached to the round bottom flask for heating. The flask was then heated until most of the sodium bromide had dissolved. The formation of two layers was seen and one was cloudy while the other one was not. The top layer was the alkyl bromide and the bottom layer 1-bromobutane. The flask was heated under reflux for 45 minutes. Simple distillation assembly was used for the codistillation of 1-bromobutane. Distillation collected was in a beaker that was immersed into an ice bath.. Distillate is put into a seperatory funnel with water(20mL) for extraction. Once layers are separated, mixture is extracted with 2M aqueous sodium hydroxide(10mL) and washed with water(20mL). Both organic and aqueous layers are taken out, and the organic layer that is 1-bromobutane is placed into a small Erlenmeyer flask and dried by anhydrous sodium sulfate until distillate isn’t sticking onto flask. After decanting the product 1-bromobutane, it is then weighed (8.478g) to calculate the percent yield(61%). With the use of the test tube method 1-bromobutane’s boiling point is determined(96°C).
2-Chloro-2-methylbutane formed by SN1 reaction
In a seperatory funnel(125mL), one must add 0.25mol of 2-methyl-2-butanol(27.5mL) and 0.75mol hydrogen chloride(62.5mL) from 12M hydrochloric acid and is swirled without a stopper on the funnel. Once solutions have been mixed, add stopper and shake, invert and release any excess pressure by opening the stopcock. Shaking and mixing until funnel has been vented should be done. Seperatory funnel is placed on ring stand with the separation of two layers, an organic and aqueous, in which the aqueous layer is removed. Once removed extraction technique is done again separately with sodium chloride(25mL), saturated sodium bicarbonate(25mL), water(20mL) and lastly with saturated sodium chloride(20mL). With the last extraction of sodium chloride the extracted 2-chloro-2-methylbutane was dried with the addition of anhydrous sodium sulfate. The decanted 2-chloro-2-methylbutane weighed (22.064 g) with the calculated percent yield of(82%). The boiling point is obtained by using the reflux in a test tube method(83°C).
References
1 Wade, L.G. Jr.; Organic Chemistry, 8th edition; Pearson: Glenview, Illinois, 2013; 232-256.
2 Ingold, Christopher K.; Structure and mechanism in organic chemistry, Cornell University Press: Ithaca, New York, 1953; Chapter 4.
3 Carey, Francis A.; Sundberg, Richard J. Advanced Organic Chemistry Part A: Structure and Mechanisms, 5th edition; Springer: Spring Street, New York, 2007; Chapter 4.
4 Hine, Jane; Rate and Equilibrium in the Addition Electrophile Carbon and in SN1 Reaction. Department of Chemistry of Ohio State University: Colombus, Ohio, 1997; 3701.
5 Stemp, Eric; Organic Chemistry Laboratory Manual, Mount St. Mary’s College: Los Angeles, California, 2012; 144-156.
6 Sang Kyu Lee, Dong Ju Lee, Gyu Sub Ko, Se Hyun Yoo, Hyun Woo Ha, Mi Jeong Kang, Tae Cheon Jeong; Role of glutathione conjugation in 1-bromobutane-induced hepatotoxicity in mice, Food and Chemical Toxicology, Yeungnam University: South Korea,Volume 48, Issue 10, October 2010, Pages 2707-2711.
7 A. B. Arbuzov, V. A. Drozdov, M. O. Kazakov, A. V. Lavrenov and M. V. Trenikhin.; In Situ Study of the Interaction between tert