Identification of an Unknown Carbonyl
Identification of an Unknown Carbonyl
By: Stacey Walker
Abstract
The 2,4-dinitrophenylhydrazine (2,4-DNPH) and semicarbazone derivatives of the unknown carbonyl are synthesized and their melting points are obtained. The infrared (IR) and proton nuclear magnetic resonance (NMR) and boiling range of the liquid unknown is obtained. Based on boiling point and infrared and proton nuclear magnetic resonance, the derivative is 2,4-pentanedione.
Introduction
In this experiment, the 2,4-dinitrohenylhydrozine (2,4-DNPH) and semicarbazone derivatives of an unknown carbonyl (#55) is synthesized. The melting range of these derivatives as well as the boiling point of the liquid unknown is obtained to narrow down the list of candidates the unknown can be. The infrared (IR) and nuclear magnetic resonance (NMR) spectra of unknown carbonyl is obtained. These spectra are used to determine the identity of the unknown from the narrowed list of possible unknowns. The results show that the unknown carbonyl is either 2,4-pentanedione.
Formation of 2,4-DNPH derivative of 2,4-pentanedione:
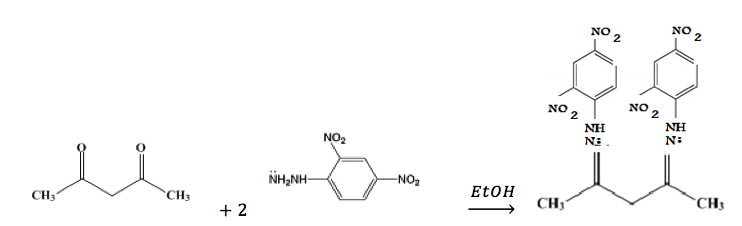
Formation of semicarbazone derivative of 2,4-pentanedione:
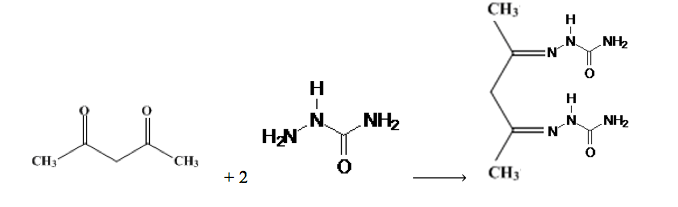
Experimental Section
Infrared (IR) spectra were collected using an IR spectrophotometer. The IR spectroscopy involved the accumulation of background spectra, which were subtracted from the spectra of the unknown sample to provide the response of the sample. Each experiment resulted from the average 8 scans, with the background being the accumulation of 8 spectra scans without the sample present. The resolution of the spectrometer was set at 4 cm-1 for all spectra. Proton nuclear magnetic resonance (NMR) spectra were recorded on a spectrometer. The NMR spectroscopy involved mixing the unknown and carbon tetrachloride in a 1:1 ratio and adding 2-3 drops of the chemical shift standard, tetramethylsilane (TMS).
The 2,4-dinitrohenylhydrozone (2,4-DNPH) derivative of the unknown was synthesized. In a test tube, 0.5 mL of the unknown and 20 mL of 95% ethanol were mixed. Fifteen mL of 2,4-
Experimental Section (cont.)
DNPH reagent was then added to the mixture in a test tube and shaken vigorously. The resulting red-orange precipitate was suction filtered and washed with cold water.
The semicarbazone derivative of the unknown was synthesized. In a 50 mL Erlenmeyer flask, 0.5 mL of the unknown and 5 mL of the semicarbazone reagent were combined and shaken vigorously. The white semicarbazone derivative formed immediately and was suction filtered and washed with cold water.
The melting ranges of both derivatives were obtained using a melting point apparatus. A simple distillation was carried out to determine the boiling range of unknown.
Results and Discussion
The boiling range of the unknown was found to be approximately 148˚C to 154˚C. On the unknown list provided, this observation narrows the list of possible unknowns to 2,4-pentanedione, 4-heptanone, 3-heptanone, 2-heptanone, heptanal, or cyclohexanone. The melting range of the 2,4-DNPH derivative was 136.0˚C to 137.2˚C and the melting range of the semicarbazone derivative was 183.0˚C to 187.0˚C. Neither of the derivatives melting ranges matched the provided literature, so the list of possible unknowns was not able to be further narrowed without the use of IR and NMR data.
The data obtained from the IR spectroscopy, shown in the table below, limited the list of possible unknowns to 2,4-pentanedione, 4-heptanone, 3-heptanone, 2-heptanone, or cyclohexanone. On the IR spectra taken for the liquid unknown, there were prominent peaks at 1359.97 cm-1, 1376.06 cm-1, 1428.61 cm-1, 1449.58 cm-1, 1712.24 cm-1, 28.61.36 cm-1, and 3932.45 cm-1. Peaks between 2850-2960 cm-1 and 1350-1470 cm-1 showed that the unknown contains C-H bonds. The peak at 1712.24 cm-1 showed that the unknown contains the C=O bond of a ketone. The lack of a peak near 2720 cm-1 showed that the C=O bond is part of a ketone, not an aldehyde.
Results and Discussion (cont.)
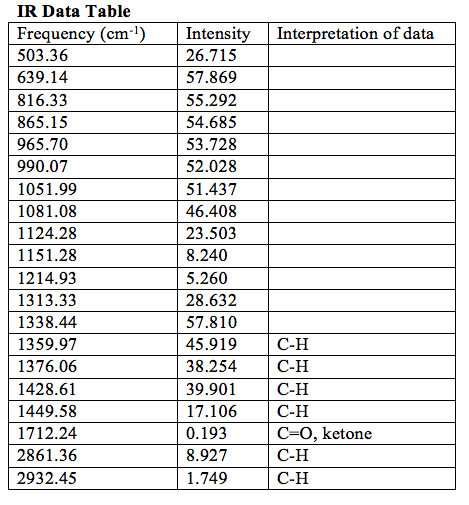
On the attached proton NMR spectra, there were peaks between 0.5 parts per million (ppm) and 2.5 ppm, with peaks at approximately 2.0 ppm and 0.94 ppm. Carbonyl hydrogens were typically found in the 2 to 2.7 ppm range. The lack of a peak near 9 and 10 further indicated that the unknown was not an aldehyde. Primary hydrogens were usually found in near 0.9 ppm (Sturm, 2014). It is possible, however, that the hydrogens close to the carbonyl group were shifted further from the standard because the hydrogens were deshielded by the carbonyl group. The peak to the left of the spectra had a larger area under the curve, showing that there are more deshielded hydrogens in the unknown. Based on these observations, one can conclude that the unknown is 2,4-pentanedione.
NMR Data Table
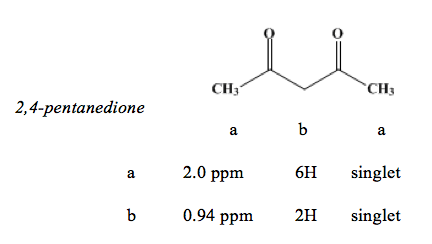
Questions
- An unknown organic compound (b. 212-216 oC) gives a positive 2,4-DNPH test and is positive with Tollen’s reagent. A semicarbazone derivative is made that melts at 228-232o What is the identity of the unknown? What would you do next?The unknown, based on the unknown list provided, would be 2-chlorobenzaldehyde. The unknown’s boiling range and semicarbazone derivative’s melting range match that of 2-chlorobenzaldehyde and a positive with Tollen’s reagent signifies that the unknown is an aldehyde. What I would do next is confirm this choice by testing for a halogen and then take the IR and NMR of the unknown.
- Predict the products of the reaction of the following with silver nitrate in ammonium hydroxide: cylcohexanone, formaldehyde, acetone, acetophenone, and butyraldehyde.
Firstly, the silver nitrate in ammonium hydroxide react and form Tollen’s reagent:

Cyclohexanone will not react because ketones are not readily oxidized and will therefore not react with the Tollen’s reagent.
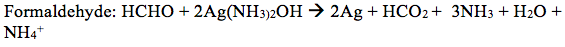
Acetophenone will not react because ketones are not readily oxidized and will therefore not react with the Tollen’s reagent.
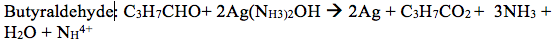
3. In the reaction of an aldehyde or ketone with derivatives of ammonia, the reaction can be catalyzed by sulfuric acid. However, it is important that the pH not be too low since the reaction will slow down at very high acid concentrations. Explain.
The acid protonates the carbonyl group and makes it more electrophilic. If there is a high acid concentration, most of the carbonyl will be protonated and it will not be as electrophilic.
4. Ketones do not oxidize readily. However, cyclohexanone will react with powerful oxidizing agents at high heat to adipic acid (HO2C-(CH2)4-CO2H). The reaction is not really one of the ketone, but the enol. Write equations to show how this is possible.
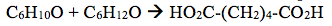
References
- Sturm, N. 2014. Spectroscopy Tables (IR & nmr). Chemistry 312 Course Handout.
California State University Dominguez Hills, Carson, CA.
http://www.nbs.csudh.edu/chemistry/faculty/nsturm/CHE310/CHE312Syllabus.htm
