Investigating the Synthesis and Reactivity of Ferrocene
Written by Dan
Abstract
In this experiment, ferrocene was synthesised and purified by vacuum sublimation. The redox behaviour of ferrocene was investigated, and it was found that the only common redox product was the blue ferrocenium ion and that it was possible to reduce ferrocenium back to ferrocene. A sample of ferrocene was acetylated, and the products were separated by flash column chromatography to give unreacted ferrocene and monoacetylated ferrocene.
Introduction
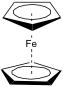
Ferrocene is a cyclopentadienyl iron (II) complex with the formula (η5-Cp)2Fe, which is diamagnetic, and crystallises to an orange/yellow solid. In the formula, η5 indicates that five atoms of the Cp ligand are coordinating to the iron ion. In the gas phase, the two cyclopentadienyl rings are eclipsed, but the solid exists in several phases in which the rings are co-parallel but in different orientations. This is due to the low barrier to rotation at ambient temperature, which is so low that there is even rotation in the solid state after crystallisation. Adding substituents onto the cyclopentadienyl rings raises this barrier to rotation, for example, the two rings will be staggered in (η5-C5Me5)2Fe.[1]
Ferrocene was discovered by accident while trying to form amines using an iron based catalyst by Sam Miller during the late 1940s but he did not submit a paper about the mysterious, orange compound he had discovered until 1951[2]. It was also discovered independently by Kealy and Pauson in 1951 in an attempt to synthesis fulvalene who published a paper in the December 15th edition of Nature in that year. Since then, its structure has been determined to be the sandwich compound shown in fig. 1.
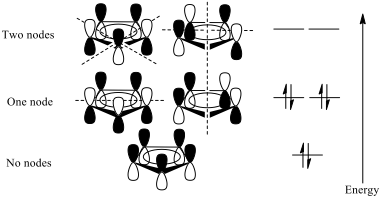
The cyclopentadiene (Cp) ion has a p orbital perpendicular to the ring at each carbon, which can produce five molecular orbitals of varying energies, filled by the six electrons in the cyclopentadienyl ring (fig. 2). The molecular orbitals of two Cp ligands can be considered together to produce eleven SALCS (symmetry adapted linear combinations), nine of which form bonding and antibonding combinations with the central iron ion’s 3d, 4s and 4p orbitals (fig. 3).
With iron in the +2 oxidation state contributing six electrons, and the Cp rings contributing six electrons each from their π-systems, there is a total valence electron count of 18, meaning that each of the nine bonding combinations illustrated in fig. 3 are filled and that the complex obeys the 18 electron rule. Two of the SALCs formed are not of the right symmetry to construct bonding or antibonding combinations with any of iron’s orbitals[3].
Ferrocene is an interesting molecule because, unlike many other organometallic compounds, it is very stable at room temperature and was one of the first organometallics to be synthesised. It is resistant to catalytic hydrogenation and Diels-Alder reactions[4]. Because they are so stable, it is convenient to purify many metallocenes by vacuum sublimation above 100 °C because they do not decompose under these conditions.
The cyclopentadienyl ring is formed by the deprotonation of cyclopentadiene with a base, which then reacts with iron (II) chloride to form ferrocene as shown in reaction (1).
2Cp-H + 2KOH + FeCl2 à (η5-Cp)2Fe + H2O + 2KCl (1)
This can be done with a variety of bases. In this experiment potassium hydroxide was used. This needed to be done in an inert atmosphere due to the fact that KOH is hygroscopic (readily absorbs moisture from the air). Had the reaction been done without an inert atmosphere, the KOH would have dissolved in the water that it absorbed, and subsequently would have formed a separate layer to that of the cyclopentadiene dissolved in an organic solvent, the deprotonation would not have proceeded.
An important characteristic of transition metals is that they can exist in many oxidation states. This property can give rise to many important applications, for example, as catalysts, which is why it was important to investigate the redox behaviour of ferrocene. A sample of ferrocene will be treated with iron (III) chloride (a weak oxidant), which should oxidise the ferrocene (Fc) to the ferrocenium ion (Fc+), as in reaction (2).
Fc + FeCl3 à Fc+Cl– + FeCl2 (2)
The ferrocenium ion is a stable ion with iron in the +3 oxidation state. It is paramagnetic and is blue in colour[5]. To determine whether or not this oxidation is reversible, ascorbic acid (a strong reducing agent, used in biological systems to reduce harmful and reactive radicals) will be added to the mixture along with some CH2Cl2 (DCM). DCM is added to the solution to separate the products of the reaction. If ferrocene is reformed, then it will readily dissolve in the DCM layer (and ascorbic acid will remain in the aqueous layer). To determine whether ferrocene can be further oxidised, it will be treated with a stronger oxidising agent, silver nitrate.
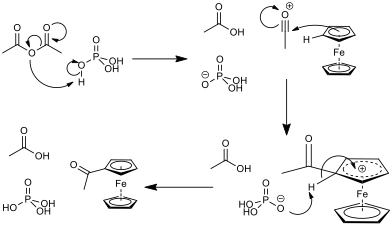
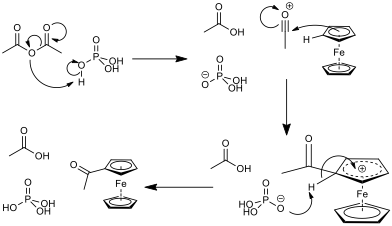
The cyclopentadienyl rings are aromatic (they are planar, cyclic, conjugated and obey Hückel’s 4n+2 rule[†]). As such, they react in a Friedel-Crafts-like electrophilic aromatic substitution. This reaction requires a carbonyl compound and an acid catalyst. In reaction acetic anhydride was used with phosphoric acid as a catalyst. The mechanism for this reaction can be seen in fig. 4. In general, the electrophilic acyl substitution of the cyclopentadienyl ring in ferrocene is much quicker than that of the same reaction with benzene, which leads to the conclusion that there is more electron density in the rings of the sandwich compound than in benzene[6]. A CaCl2 drying tube will be used to protect the reaction because acetic anhydride is moisture sensitive, and will degrade to acetic acid.
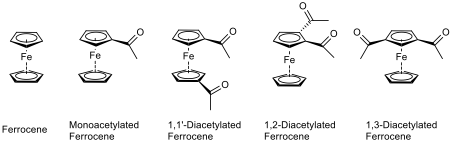
In some cases, the monoacetylated ferrocene could over-react to the diacetylated ferrocene, as well as there being some unreacted ferrocene in the reaction mixture. This means that there is potential for there to be five compounds in the reaction mixture at the end of the reaction, as shown in fig. 5.
These compounds can be separated by flash column chromatography, on the basis that each compound is different in polarity, and less polar molecules elute first since there is less attraction to the silica slurry in the column. Ferrocene is the least polar compound, because there are no polar groups in the compound. Monoacetylated ferrocene is the second least polar compound of the five because it has one polar carbonyl function.
Each of the diacetylated ferrocenes have two carbonyl functions within their structures, but the way in which they are orientated gives rise to different levels of polarity. If the two carbonyls are close to each other then there will be a high electron density in the compound around this area, whereas if the carbonyls are far away from each other, then the charge is spread out. As such, the 1,1’-diacetylated ferrocene would elute from a chromatography column first, followed by the 1,3-diacetylated ferrocene, and with 1,2-diacetylated ferrocene eluting last. The products can then be identified using IR and NMR spectroscopy, as explained in table 1.
|
Compound |
Strong peak between 1600-1700 cm-1 in IR spectrum |
Proton environments |
Splitting pattern expected in 1H NMR |
Number of protons responsible for each peak |
|
Ferrocene |
No |
1 |
Singlet |
10 |
|
Monoacetylated ferrocene |
Yes |
4 |
Singlet Triplet Triplet Singlet |
5 2 2 3 |
|
1,1’-diacetylated ferrocene |
Yes |
3 |
Singlet Triplet Triplet |
6 4 4 |
|
1,2-diacetylated ferrocene |
Yes |
4 |
Singlet Doublet Triplet Singlet |
5 2 1 6 |
|
1,3-diacetylated ferrocene |
Yes |
4 |
Singlet Singlet Singlet Singlet |
5 1 2 6 |
Table 1 – prediction of the appearance of IR and NMR spectra for ferrocene and acetylated ferrocenes
All of the compounds except ferrocene exhibit a strong absorption between 1600 and 1700 cm-1 in the IR spectrum, indicating the presence of a carbonyl function in within the molecule. From their structures, the number and multiplicity of peaks in the NMR spectrum for each compound can be predicted. Each compound produces a unique spectra, thus each can be identified.
Acetic anhydride with phosphoric acid was used to acetylated ferrocene as opposed to the more common reagents used in a Friedel-Crafts reaction of aluminium chloride with acetyl chloride in DCM because, with acyl chloride, the tendency is to over-react to the diacetylated form, and the solvent is much more harmful. Acetylation with acyl chloride also requires anhydrous conditions for a successful reaction, which is not required when using acetic anhydride[7].
In summary, the aims of this experiment were to successfully synthesise and purify the sandwich compound ferrocene, investigate its redox behaviour, and to attempt a Friedel-Crafts Acetylation in the cyclopentadienyl rings of ferrocene and separate and identify the components of the mixture using flash column chromatography and IR and NMR spectroscopy.
Experimental
Synthesis and Purification of Ferrocene
A 3-necked round bottom flask was equipped with a nitrogen inlet and a stopper. After nitrogen was used to flush out the apparatus, crushed potassium hydroxide (8.05 g, 0.143 mol) and 1,2-dimethoxyethane (20 cm3) was added with stirring. Cyclopentadiene (1.8 cm3, 0.021mol) was added against the flow of nitrogen. The suspension was stirred for ten minutes during which time the solution went from being colourless to beige, then a darker brown colour before adding a solution of iron (II) chloride tetrahydrate (2.04g, 0.0103 mol) in DMSO (8 cm3) over a period of 20 minutes through a pressure equalising dropping funnel with vigorous stirring. When the addition was complete, the mixture was stirred for a further 30 minutes during which time the solution darkened further to a very dark brown colour.
The reaction mixture was then disconnected from the nitrogen flow and poured onto a slurry of aqueous HCl (30 cm3, 6 moldm-3) and ice (30 g). After the slurry had been stirred for 15 minutes, a yellow precipitate (crude ferrocene) was collected by vacuum filtration and washed with water (4 x 10 cm3). The filtrate was blue coloured. Tin (II) chloride (4 heaped spatulas) was added to the filtrate which caused an orange solid to precipitate out of solution and was combined with the rest of the crude ferrocene (0.56 g). The crude product was dried on a steam bath to give orange crystals and purified by vacuum sublimation between 100-120 °C which to give ferrocene (0.14 g; 7.4 %; mp 168 – 170 °C).
Investigation of the Redox Behaviour of Ferrocene
Fresh ferrocene (100 mg) was dissolved in acetone. Iron (III) chloride hexahydrate (0.1 g) was added to water (5 cm3). Both of these solultions were orange. Half of the ferrocene solution was mixed with the iron (III) chloride solution, during which, the solution turned a dark blue colour.
This blue solution was then treated with a small spatula of ascorbic acid with shaking. DCM was added to give a discernable second layer with shaking. During the reaction, the solution was green, and after the reaction was complete the lower organic layer was orange and the upper aqueous layer was colourless.
A few drops of silver nitrate were added to the original acetone solution of ferrocene. During the reaction, the solution went from orange to green to blue, and a grey precipitate was formed.
Acetylation of Ferrocene
Orthophosphoric acid (0.25 cm3, 85 %) was added dropwise with constant stirring to a mixture of ferrocene (0.2563 g, 1.38 mmol) and acetic anhydride (5 cm3, 0.055 mol). The mixture was protected using a CaCl2 drying tube and heated in a vigorously boiling water bath for ten minutes. The mixture was poured onto ice (19 g), and when it had melted the solution was neutralised with solid NaHCO3 until no more gas was evolved. The mixture was cooled in an ice bath for 30 minutes before removing any solid by suction filtration and air drying for 15 minutes. The solid in the sinter funnel was washed with ethyl acetate to extract the crude product. The aqueous and organic layers were separated, and the organic layer was dried over MgSO4. The solvent was removed from the organic layer until ca. 1 cm3 remained. This solution was kept in the freezer for one week.
To assess the required solvent system required for the mobile phase of the chromatography column, a sample of pure ferrocene, acetylated ferrocene, and a mixture of the two were run alongside each other on TLC plates developed in various mixtures of solvents with differing polarities and visualised under UV light. The development solvent that gave the best separation was a 60:40 mix of 40-60 petroleum ether: ethyl acetate and that there were two compounds in the reaction mixture. Sketches of the TLC plates can be found in appendix 2.
After loading the chromatography column, it was run with a mobile phase of one hundred percent 40-60 petroleum ether, and as the first fraction (a yellow/orange solution) began to elute, the polarity of the eluent was increased by increasing the proportion of ethyl acetate. This was done slowly, raising the solvent system polarity in 10% steps until the solvent mixture was a 60:40 mix of 40-60 petroleum ether: ethyl acetate and the second fraction (a red/orange solution) had eluted. TLC analysis of the collected eluate can be found in appendix 3.
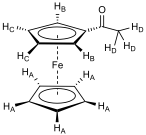
After maximising solvent polarity (100% ethyl acetate) a pale pink layer remained at the top of the silica column, with a light turquoise layer slightly below it. Combining appropriate samples that had eluted and removing the solvent gave two products. The compound that eluted first was unreacted ferrocene (0.09 g, 35.1 % unreacted), a light orange crystalline solid (mp 173 – 174 °C; δH(250MHz; CDCl3) 4.3 (10H, s, C5H5)), followed by monoacetylated ferrocene (0.09 g; 28.6 %), a dark orange crystalline solid (mp 83 °C; νmax/cm-1 (DCM solution cell) 3065.8 (CH str, sp2), 2988.8 (CH str, sp3), 1667.0 (C=O), 1607.5 (H2O in DCM), 1454.2 (C=C aromatic), 1379.6 (CH bend); δH(400MHz; CDCl3) 4.8 (2H, t, J 2.0 Hz, HB), 4.5 (2H, t, J 2.0 Hz, HC), 4.3 (5H, s, HA), 2.4 (3H, s, HD)).
Where a proton is assigned a subscript letter, it is in reference to the structure in fig. 6.
Results and Discussion
Synthesis and Purification of Ferrocene
After the initial collection of crude ferrocene, the filtrate that passed through the Buchner funnel was blue in colour. This suggested the presence of the ferrocenium ion. Tin (II) chloride was added to the mixture to act as a reductant, such that the iron (III) in ferrocenium was reduced down to iron (II) and ferrocene precipitated out of the solution.
The experimental melting point of the sublimed ferrocene was 168 – 170 °C, which is low compared to the documented melting point (174 °C[8]), suggesting impurity in the sample, even though vacuum sublimation was used to purify the specimen, thus implying that the impurity has a similar vapour pressure to that of ferrocene at reduced pressure between 100 and 120 °C. The impurity may have been derived from the dimer of cyclopentadiene.
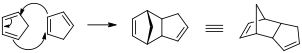
At room temperature, cyclopentadiene can slowly dimerise via a Diels-Alder mechanism to give the product bis(cyclopentadiene). Fig 7 shows the mechanism for the formation of, and the structure of bis(cyclopentadiene). This compound has a documented melting point of 33 °C when pure[9], so at ambient temperatures and pressures it exists as a solid. There may have been enough dicylopentadiene in the solid to depress the melting point of the crystals.
The yield of the reaction was only 7.4 %. This may have been due to the fact that some of the cyclopentadiene had dimerised before reacting with potassium hydroxide to form the cyclopentadienyl anion. As such, the yield of ferrocene was depressed.
Investigation of the Redox Behaviour of Ferrocene
When ferrocene was treated with iron (III) chloride, the orange solution turned blue. Iron (III) chloride is a weak oxidant, and the iron in the complex can gain an electron to be reduced to the +2 oxidation state. Reactions (3) and (4) show the half equations for this reaction.
FeCl3 + e– à FeCl2 + Cl– (3)
Fc à Fc+ + e– (4)
The transfer of an electron from the ferrocene to the iron chloride complex oxidises the ferrocene to ferrocenium. This ion has a characteristic blue colour. The increased charge on the iron in ferrocenium will have attracted the negative ligands closer to the nucleus, and as such, increase the splitting energy between the HOMO and LUMO. This increase in splitting energy means that photons of a higher energy (shorter wavelength) would be required to promote electrons from the HOMO to the LUMO. Absorption towards the high energy infrared region of the electromagnetic spectrum means that light with wavelength closer to the ultraviolet end of the electromagnetic spectrum (blue and purple colours) passes through the sample and is observed.
On the addition of ascorbic acid, the reaction mixture appeared green for some of the reaction. The green colour is likely to be the result of mixing of some blue compound with some orange compound, since the resultant colour of the mixture was orange. Once the organic and aqueous layers had separated, the orange colour was contained within the lower DCM layer. Ferrocene is only soluble in organic solvents; leading to the conclusion that ferrocenium had been reduced back to ferrocene and had dissolved in the DCM layer.
On mixing ferrocene with silver nitrate, a much stronger oxidising agent than iron (III) chloride, an orange solution became blue, suggesting that ferrocenium had once again been produced by the half equations (5) and (6).
AgNO3 + e– à Ag + NO3– (5)
Fc àFc+ + e– (6)
In equation (5) we see that elemental silver is formed from the reduction of silver nitrate which is responsible for the grey precipitate was formed during the experiment.
Acetylation of ferrocene
TLC analysis prior to running the column showed that there were only two compounds in the mixture after the ferrocene had been acetylated, and, as expected, only two separate fractions eluted from the column. The first fraction, suspected to be unreacted ferrocene, produced and NMR spectrum (appendix 5) very similar to that of the provided NMR spectrum of pure ferrocene (appendix 4). To confirm the identity of this product, a comparative TLC of this fraction was run against a sample of ferrocene from the chemical stores (appendix 3), and its melting point was tested. The comparative TLC showed that the first fraction and ferrocene had identical Rf values. The melting point of the recovered fraction was 173 – 174 °C (which is identical to the documented value of 172 – 174 °C[10]) which confirmed the identity of this fraction to be ferrocene. The second fraction to elute produced and NMR spectrum with a splitting pattern identical to that predicted in table 1 for the monoacetylated ferrocene. Its IR spectrum also showed a strong absorption at 1667 cm-1 indicating the presence of a carbonyl group. The melting point of the recovered fraction was 83 °C (matches the documented value of 81 – 83 °C[11]), confirming that the second fraction was monoacetylated ferrocene.
After the both products had eluted, there were still two layers close to the top of the column. There was a pale pink layer and a light turquoise layer. The turquoise layer may have been due to the oxidation of some unreacted ferrocene to the ferrocenium ion (which is blue in colour). Because the ferrocenium ion is a charged particle, it will have been very attracted to the silica in the column and it would have required a much more polar solvent than ethyl acetate to remove this from the column (perhaps DMSO or acetonitrile). The pink layer was due to staining of the silica by the products obtained.
The yield of monoacetylated ferrocene was 28.6 %. This may have been low if some of the ferrocene was oxidised to ferrocenium, in which case, there would be less ferrocene available for the reaction with acetic anhydride. Acetic anhydride can also react with moisture in the air to form acetic acid. If this process had occurred before fixing the CaCl2 drying tube to the reaction vessel, then there would have been less acetic anhydride to react with ferrocene. Heat also contributes to the instability of acetic anhydride, and since the reaction was performed in a boiling water bath, some of the acetic anhydride may have decomposed before getting a chance to react with the ferrocene.
In the introduction it was explained that monoacetylated ferrocene may have reacted with acetic anhydride again to produce diacetylated ferrocene. In this reaction, no diacetylated ferrocene was collected, and the production of monoacetylated ferrocene was the preferred route. This may have been due to a lack of acetic anhydride to react with after monoacetylation had taken place, which would also account for the large amount of unreacted ferrocene recovered from the column.
Conclusion
There were three aims of this experiment, with the first being to synthesise and purify ferrocene. The melting point of the acquired substance suggested that ferrocene was present in the majority; however there were still some minor impurities, which caused the depression of the melting point.
The second aim was to investigate the redox properties of ferrocene. It was found that, irrespective of the strength of the oxidising agent, ferrocene could only be oxidised to the ferrocenium ion (iron is oxidised from the +2 to the +3 oxidation state), and that this oxidation was reversible (ferrocenium can be reduced back to ferrocene), however, no further reduction was possible.
The final aim of this experiment was to attempt a Friedel-Crafts Acetylation to make monoacetylated ferrocene and to separate and identify the products. From analysis of spectral data and by comparison of the melting points of the crystals formed, it was confirmed that of the two fractions that eluted from the column, the first was unreacted ferrocene, and that the second was monoacetylated ferrocene. No diacetylated ferrocene was yielded from this reaction. The yield of monoacetylated ferrocene was quite low, and to improve this, more acetic anhydride could have been used to ensure that it does not decompose before it can react with ferrocene.
[†] Hückel’s 4n+2 rule states that there must be 4n+2 electrons in a π-system for it to be aromatic, where n has any integer value
[1] CE Housecroft, AG Sharpe, Inorganic Chemistry, third edition, 2008, Pearson Education Limited, Edinburgh
[2] Robert Burns Woodward, Architect and Artist in the World of Molecules, Chemical Heritage Foundation, Philadelphia, USA, 2001, p. 115
[3] http://alpha.chem.umb.edu/chemistry/ch611/documents/Lec14PiBondingLigands2_002.pdf A lecture on π Bonding Ligands in Transition Metal Chemistry
[4] Gary O Spessard, Gary L Meissler, Organometallic Chemistry, second edition, 2010, Oxford University Press, New York, p 6
[5] CE Housecroft, AG Sharpe, Inorganic Chemistry, third edition, 2008, Pearson Education Limited, Edinburgh
[6] Gary O Spessard, Gary L Meissler, Organometallic Chemistry, second edition, 2010, Oxford University Press, New York, p 116
[8] http://msds.chem.ox.ac.uk/FE/ferrocene.html; last updated September 2005
[9]http://www.sigmaaldrich.com/catalog/ProductDetail.do?D7=0&N5=SEARCH_CONCAT_PNO|BRAND_KEY&N4=454338|ALDRICH&N25=0&QS=ON&F=SPEC