Kinetic and Thermodynamic Control of a Reaction
By: Ashley Martin
Introduction
This experiment explored the concepts of thermodynamic and kinetic products and controlling the formation of each product of the reaction of semicarbazide hydrochloride and cyclohexanone as well as semicarbazide hydrochloride and 2-furaldehyde. The kinetic product is formed under conditions where reactions that require a lot of energy are irreversible or in which there is no equilibrium achieved. The amount of product is determined by their rates of formation, which correlates to the height of the transition state on an energy diagram. A product with a low transition state requires low energy and is made faster under conditions of kinetic control. The reverse reaction can occur easily, and the major product of the reaction under these conditions is the kinetically controlled product. Under thermodynamic control, conditions are such that equilibrium can be established between the products and the reactions. The major product is the thermodynamically controlled product. The amount of each product made is related to the stability of each product. The more stable product is formed as the majority. The activation energy of the reverse reaction is high and this makes it harder for the reverse reaction to occur.
The products formed were analyzed using melting point ranges in order to determine which products were under kinetic control and which were under thermodynamic control, as well as the relative stabilities of the products, as compounds with higher melting points are more stable because it takes more energy to break their bonds.
pH also affects the rate of reaction, and because the rate determining step is the nucleophilic attack of the semicarbazide on a carbonyl carbon, the pH greatly affects the reaction. A buffer system is created in this experiment with a pH of 6.1-6.2. The reaction is slow in this range making it easier to differentiate between thermodynamic and kinetic control.
Methods/Procedures
Part A Preparation of Cyclohexanone Semicarbazone
.5g of semicarbazide hydrochloride and 1.06g of dibasic potassium phosphate in 6mL of water in a 25-mL Erlenmeyer flask. .5mL of cyclohexanone and 2.5mL of 95% ethanol was prepared in a test tube. The ethanolic solution was added to the aqueous semicarbazide solution with stirring. The solution was allowed to crystalize for 10 minutes, allowing for formation of the semicarbazone. The crystals were collected by vacuum filtration. The mass of the collected cyclohexanone semicarbazone was taken with tarred instruments. The melting point of the collected product was also taken.
Figure 1. Mechanism of the Buffer System for Parts A, B, C and E
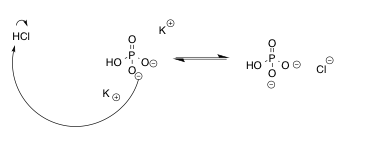
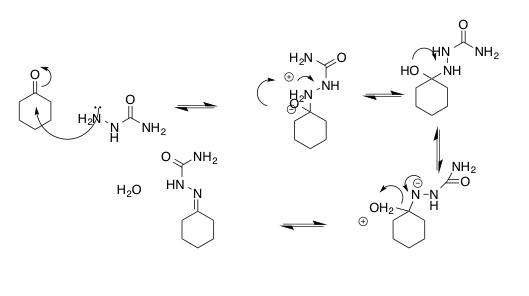
Figure 2. Mechanism of the reaction of semicarbazide with cyclohexanone to form cyclohexanone semicarbazone and water
Part B Preparation of 2-Furaldehyde Semicarbazone
.5g of semicarbazide hydrochloride and 1.06g of dibasic potassium phosphate in 6mL of water in a 25-mL Erlenmeyer flask. .5mL of 2-furaldehyde and 2.5mL of 95% ethanol was prepared in a test tube. The ethanolic solution was added to the aqueous semicarbazide solution with stirring. The solution was allowed to crystalize for 10 minutes, allowing for formation of the semicarbazone. The crystals were collected by vacuum filtration. The mass of the collected 2-furaldehyde semicarbazone was taken with tarred instruments. The melting point of the collected product was also taken.
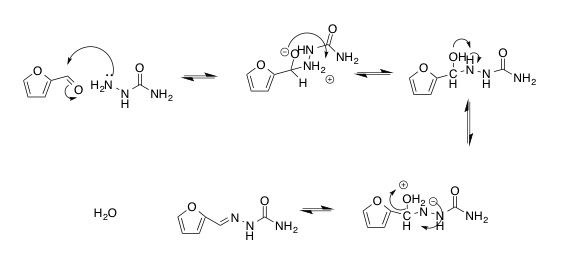
Figure 3. Mechanism of the preparation of 2-furaldehyde semicarbazone
Part C Reactions of Semicarbazide with Cyclohexanone and 2-Furaldehyde in Phosphate Buffer Solution
3.03 g semicarbazide hydrochloride and 6.0g dibasic potassium phosphate were dissolved in 75mL water, denoted as solution W. 3.0mL of cyclohexanone and 2.5mL of 2-furaldehyde were dissolved in 15mL of 95% ethanol, denoted as solution E. 25mL of solution W and 5mL of solution E were cooled to 0-2°C. Solution E was added to solution W, allowing crystals to form. The crystals were cooled for 5 minutes and collected through vacuum filtration. The crystals were massed using tarred instruments and the melting point range was taken.
A 5mL portion of solution E was added to 25mL of solution W at room temperature, and the solution was allowed to stand for 5 minutes before cooling in an ice bath. The crystals were collected through vacuum filtration, massed using tarred instruments, and their melting point was taken.
25mL of solution W and 5mL of solution E were warmed to 80°C. Solution E was added to solution W with stirring. The solution was heated for 10 minutes, then cooled to room temperature and cooled in an ice-water bath for 10 minutes. The crystals were collected through vacuum filtration, massed using tarred instruments and their melting point was taken.
Part D Reactions of Semicarbazide with Cyclohexanone and 2-Furaldehyde in Bicarbonate Buffer Solution
2.03g semicarbazide hydrochloride and 4.03g sodium bicarbonate were dissolved in 50mL water. 2.0mL cyclohexanone and 1.6mL of 2-furaldehyde were dissolved in 10mL 95% ethanol. The solutions were divided into 2 equal portions. One half of each solution were mixed and allowed to stand for 5 minutes. The crystals were collected using vacuum filtration, massed using tarred instruments and their melting point was taken.
The other halves of each solution were warmed separately to 80°C. They were combined and heated for 10 minutes. The solution was cooled to room temperature, then cooled in an ice water bath. The crystals were collected through vacuum filtration, massed using tarred instruments and their melting point was taken.
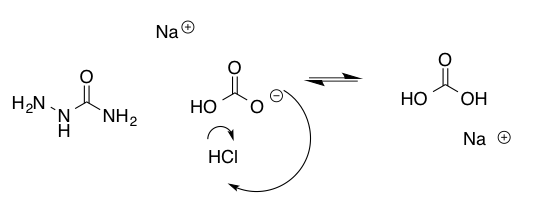
Figure 4. Mechanism of Buffer System for Part D
Part E Tests of Reversibility of Semicarbazone Formation
.3g cyclohexanone semicarbazone from part A, .3mL of 2-furaldehyde, 2mL of 95% ethanol and 10mL water were combined in a 25-mL Erlenmeyer flask. The mixture was warmed until homogeneous, and then heated for 3 minutes. The solution was cooled to room temperature and then placed in an ice bath. The crystals were collected using vacuum filtration, massed using tarred instruments and the melting point was taken.
.3g 2-furaldehyde semicarbazone from part B, .3mL cyclohexanone, 2mL of 95% ethanol and 10mL water were combined in a 25-mL Erlenmeyer flask. The mixture was warmed until homogeneous, and then heated for 3 minutes. The solution was cooled to room temperature and then placed in an ice bath. The crystals were collected using vacuum filtration, massed using tarred instruments and the melting point was taken.
Results
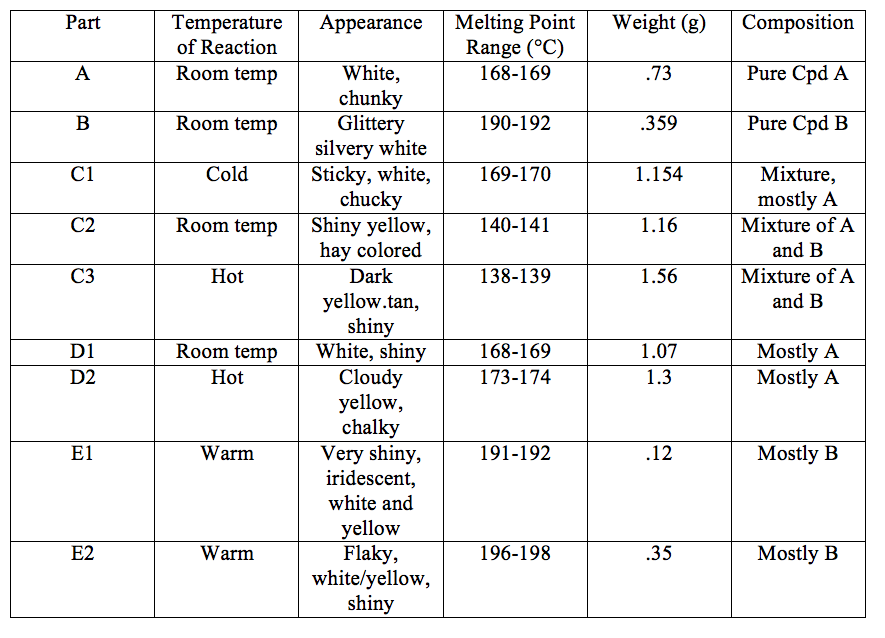
Discussion and Interpretation of Results
Part A yielded .0047mol of Cyclohexanone Semicarbazone, and part B yielded .0023mol of 2-Furaldehyde Semicarbazone. Based on the experimental data, the cyclohexanone semicarbazone is likely the kinetic controlled product and the thermodynamically controlled product is B. In part C1, the product was formed very quickly, and the melting point was close to the melting point of product A. The quick formation indicates kinetic control. In C2, the crystals took a moderate amount of time to form. The melting point was very low, and this indicates a mixture of both. C3 had the longest reaction time, and this was with heating to add energy to the reaction. This should have created B in majority because it was the more stable product, but instead created a mixture of A and B.
Part D was indicative of product A being under kinetic control. The reaction was done at room temperature and the melting point indicated that the product was mostly A. Part D2 should have formed mostly B, but the melting point range indicated that a mixture was formed, with more A than B. The reaction time was the longest and energy was added to the reaction, but B was not formed as a majority.
Part E should demonstrate which product was kinetically controlled and which was controlled thermodynamically. The kinetically controlled product should be able to complete the reverse reaction with a decent yield, as the activation energy of the reverse reaction is not very large. The thermodynamically controlled product is the more stable product, and so the yield of the reverse reaction of the thermodynamically controlled product. E1 should have a lower yield than E2, which was demonstrated in our experiment.
Figure 5. Potential Energy vs Reaction Progress showing Activation Energies
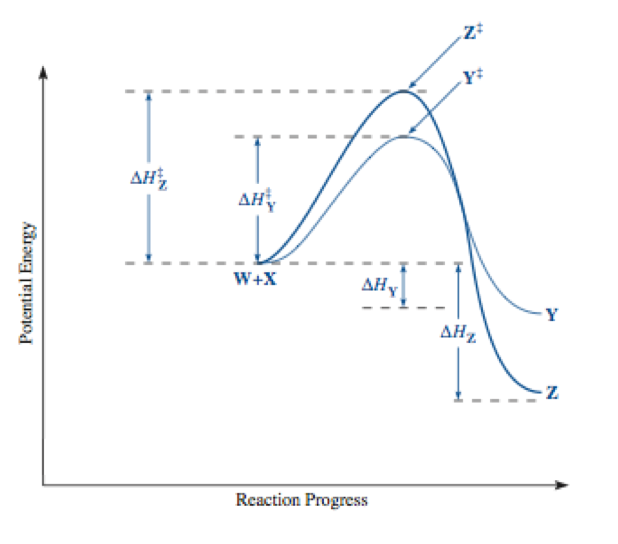
Key:
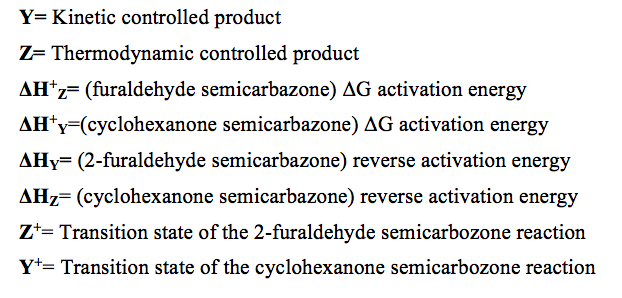
Conclusions:
This experiment was successful in demonstrating the relationship between kinetic and thermodynamic control. This was demonstrated through the use of melting point analysis of compounds formed through reactions performed in different reaction conditions. This experiment concluded that the thermodynamic controlled product was 2-furaldehyde carbazone and the kinetic controlled product was cyclohexanone semicarbazone.
References:
Gilbert, J. C., Martin, S. F., Experimental Organic Chemistry, A Miniscale and Microscale Approach, 5th ed.
