Nucleophilic Substitution
Nucleophilic Substitution
By: Nucleophillic Substitution
Introduction
Nucleophilic substitution is a process in which a leaving group on a compound is replaced by a nucleophile. There are two different types of substitution reactions. They are known as SN1 and SN2 reactions. There are many differences between these two reactions. For example, SN1 are two step reactions, involving the formation of a carbocation intermediate, followed by a nucleophilic attack. These reactions are promoted by a polar protic solvent and are favored by tertiary electrophiles. SN2 reactions involve two different species and a rate determining step. It is a one-step reaction involving a back-side attack. Because of this process, an inversion for stereochemistry occurs. These reactions are promoted by polar aprotic solvents and are favored by strong, primary electrophiles. Overall, four aspects determine whether a SN1 or SN2 path will be taken: structure of the electrophile, nucleophile strength, leaving group ability, and solvent type. A tertiary electrophile favors SN1, while a primary electrophile favors SN2. A strong nucleophile favors SN2. Weak bases are more stable, and therefore make for better leaving groups. Finally, protic solvents favor SN1, while aprotic solvents favor SN2. In this experiment, 1-chlorobutane, 1-bromobutane, 2-chlorobutane, 1-chloro-2-methylpropane, and 2-chloro-2-methylpropane are tested as substrates in SN1 and SN2 reaction scenarios to see which is more effective for each reaction.
Procedures and Observations
For the effect of the substrate on an SN1 reaction, five test tubes were obtained. 0.1 mL of 1-chlorobutane was placed in the first test tube. 0.1 mL of 1-bromobutane was placed in the second test tube. 0.1 mL of 2-chlorobutane was placed in the third test tube. 0.1 mL of 2-chloro-2-methylpropane was placed in the fourth test tube. 0.1 mL of 1-chloro-2-methylpropane was placed in the fifth test tube. 1 mL of 1% ethanolic silver nitrate was added to each of the five test tubes quickly and a timer was started. The time was recorded for when a precipitate started to form in each test tube. After five minutes, the test tubes which had no precipitate yet formed were placed in a 50˚C water bath. The timer was started again for the samples that needed heat, and the time was recorded for when a precipitate started to form in each of these samples. 1-chlorobutane did not form a precipitate, even in the presence of heat. For the effect of the substrate on an SN2 reaction, five test tubes were obtained. 0.1 mL of 1-chlorobutane was placed in the first test tube. 0.1 mL of 1-bromobutane was placed in the second test tube. 0.1 mL of 2-chlorobutane was placed in the third test tube. 0.1 mL of 2-chloro-2-methylpropane was placed in the fourth test tube. 0.1 mL of 1-chloro-2-methylpropane was placed in the fifth test tube. 1 mL of sodium iodide in acetone was added to each of the five test tubes quickly and a timer was started. The time was recorded for when a precipitate started to form in each test tube. After five minutes, the test tubes which had no precipitate yet formed were placed in a 50˚C water bath. The timer was started again for the samples that needed heat, and the time was recorded for when a precipitate started to form in each of these samples. All five samples formed a precipitate eventually, whether it was with or without heat. Finally, in order to test the effect solvent polarity has on a SN1 reaction, 0.1 mL of 2-chlorobutane was obtained and 1 mL of 1% silver nitrate in a solution of 1:1 ethanol and water was added to this test tube. No precipitate formed in the five minutes with no heat, and the sample appeared to be immiscible, so this sample was heated in a 50˚C water bath and a precipitate finally formed. After heat was added, the sample also appeared to be miscible. See Calculations and Figures for the table showing the timed results.
Discussion
Once again, four aspects determine whether a SN1 or SN2 path will be taken: structure of the electrophile, nucleophile strength, leaving group ability, and solvent type. A tertiary electrophile favors SN1, while a primary electrophile favors SN2. A strong nucleophile favors SN2. Weak bases are more stable, and therefore make for better leaving groups. Finally, protic solvents favor SN1, while aprotic solvents favor SN2. For the SN1 reaction, all of the substrates yielded a precipitate without heat except for 1-chlorobutane. For the SN2 reaction, all of the substrates yielded a precipitate, but all of them needed heat for the precipitate to form except for 1-bromobutane. This is because the appropriate solvents were used for the reactions to occur. For the SN1 reactions, the 1% ethanolic silver nitrate was used. It is protic, in that a hydrogen ion could be donated. For the SN2, an aprotic solvent was used (NaI in acetone), in that no hydrogen ion could be donated. The final reaction with 2-chlorobutane and 1% silver nitrate in a 1:1 mixture of ethanol and water was a SN1 reaction, but since the precipitate formed only with heat, the solvent was not as effective, or polar, as it was in the first part of the experiment with the initial SN1 reactions. For SN1 reactions, a polar protic solvent is best for the reaction to occur, or, a solvent in which a hydrogen ion can be readily donated (as seen in ethanolic silver nitrate). Because hydrogen bonding occurs in the 1:1 mixture of ethanol and water, there are less hydrogen ions willing to be donated to be an effective protic solvent. Heat must be added to break the hydrogen bonds, and then the hydrogen on the ethanol can be donated, making the solvent protic again, so then the SN1 reaction can occur for 2-chlorobutane. In 1-chlorobutane and 1-bromobutane, the leaving group was attached to a primary carbon, or primary electrophile. That is why these substrates were better in the SN2 reactions than the SN1 reactions. On the other hand, bromine makes for a much better leaving group in 1-bromobutane, than chlorine does in 1-chlorobutane. This is because the C-Br bond is much weaker than the C-Cl bond. Overall, this is due to electronegativity. This holds true in both SN1 and SN2 reactions. In SN1 reactions, a tertiary halide makes for the best kind of substrate. This is reinforced in the experiment because a precipitate formed very quickly for 2-chloro-2-methylpropane in the SN1 reaction. Although 1-chlorobutane, 1-bromobutane, and 1-chloro-2-methylpropane are all primary halides, 1-chloro-2-methylpropane is a hindered primary halide, which explains why the SN1 reaction still occurs with ease. In the SN2 reactions, the primary halides reacted within the shortest amount of time, such as 1-chlorobutane, 1-bromobutane (this compound being the only compound not needing to be heated), and 1-chloro-2-methylpropane. Finally, as with any reaction, heat helped the reaction proceed because it increased kinetic energy, or helped the molecules experience more collisions.
Calculations and Figures
SN1 Reaction (with 1% ethanolic silver nitrate)
| Substrate | Time for ppt to form | Heated? |
| 1-chlorobutane | N/A (no ppt formed) | Yes |
| 1-bromobutane | 55 seconds | No |
| 2-chlorobutane | 4 minutes | No |
| 2-chloro-2-methylpropane | 7 seconds | No |
| 1-chloro-2-methylpropane | 3 seconds | No |
SN2 Reaction (with NaI in acetone)
|
Substrate |
Time for ppt to form | Heated? |
| 1-chlorobutane | 28 seconds | Yes |
| 1-bromobutane | 12 seconds | No |
| 2-chlorobutane | 1 minute | Yes |
| 2-chloro-2-methylpropane | 50 seconds | Yes |
| 1-chloro-2-methylpropane | 15 seconds | yes |
SN1 Reaction (with 1% silver nitrate in a 1:1 mixture of ethanol and water)
| Substrate | Time for ppt to form | Heated? |
| 2-chlorobutane | 50 seconds | Yes |
Additional Questions
Give an example of both an SN1 and SN2 mechanism.
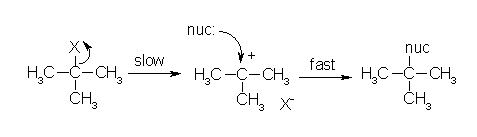
An example of an SN1 mechanism would be from the experiment in which the substrate is 2-chloro-2-methylpropane and the solvent in 1% ethanolic silver nitrate. The halide is tertiary and the solvent is polar and protic, therefore the conditions are correct for the SN1 reaction to occur. By using the picture, the X molecule can be assumed to be Cl and the nucleophile can be assumed to be nitrate. The precipitate would be AgCl. (Picture source: www.chemhelper.com)
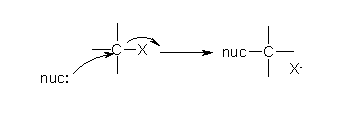
An example of an SN2 mechanism would be from the experiment in which the substrate is 1-bromobutane and the solvent is sodium iodide in acetone. The halide is primary with an excellent leaving group, and the solvent in polar and aprotic, therefore the conditions are correct for the SN2 reaction to occur. By using the picture the X molecule can be assumed to be Br, and the nucleophile can be assumed to be iodide. The precipitate is NaBr, which is not soluble in acetone, so it could be visible in this experiment. (Picture source: www.chemhelper.com)
What would be the effect of carrying out the sodium iodide in acetone reaction with the alkyl halides using an iodide solution half as concentrated?
If the iodide solution were half as concentrated, the SN2 reaction would occur at half the rate it normally would, and only half as much precipitate would form.
The addition of sodium or potassium iodide catalyzes many SN2 reactions of alkyl chlorides or bromides. Explain.
The sodium or potassium ion is very positively charged, and this can help the negatively charged leaving group actually leave. Since SN2 reactions occur in one step, if the leaving group leaves easily, then the nucleophile can attack easily.
What would happen if the anhydrous acetone were wet?
If the anhydrous acetone were wet, that would mean water molecules were present. If water molecules were present, the NaBr precipitate would dissolve in the water molecules, therefore making the precipitate unobservable and yielding erroneous results.