Periodic Laws: Newlands (Chemical Society Report) and Mendeleev
- Chemical Society Proceedings. Report on Newland’s Law of Octaves. (1866)
…Mr. JOHN A. R. NEWLANDS read a paper entitled The Law of Octaves, and the Causes of Numerical Relations among the Atomic Weights. The author claims the discovery of a law according to which the elements analogous in their properties exhibit peculiar relationships, similar to those subsisting in music between a note and its octave. Starting from the atomic weights on Cannizzaro’s system, the author arranges the known elements in order of succession, beginning with the lowest atomic weight (hydrogen) and ending with thorium (=231.5); placing, however, nickel and cobalt, platinum and iridium, cerium and lanthanum, &c., in positions of absolute equality or in the same line. The fifty-six elements so arranged are said to form the compass of eight octaves, and the author finds that chlorine, bromine, iodine, and fluorine are thus brought into the same line, or occupy corresponding places in his scale. Nitrogen and phosphorus, oxygen, and sulphur, &c., are also considered as forming true octaves.
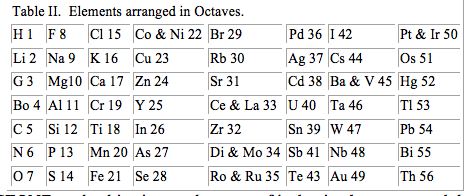
Dr. GLADSTONE made objection on the score of its having been assumed that no elements remain to be discovered. The last few years had brought forth thallium, indium, caesium, and rubidium, and now the finding of one more would throw out the whole system. The speaker believed there was as close an analogy subsisting between the metals named in the last vertical column as in any of the elements standing on the same horizontal line.
Professor G. C. FOSTER humorously inquired of Mr. Newlands whether he had ever examined the elements according to the order of their initial letters? For he believed that any arrangement would present occasional coincidences, but he condemned one which placed so far apart manganese and chromium, or iron from nickel and cobalt.
Mr. NEWLANDS said that he had tried several other schemes before arriving at that now proposed. One founded upon the specific gravity of the elements had altogether failed, and no relation could be worked out of the atomic weights under any other system than that of Cannizzaro.
2a. Dmitri Mendeleev (1834-1907): On the Relationship of the Properties of the Elements to their Atomic Weights (1869).
By ordering the elements according to increasing atomic weight in vertical rows [see below] so that the horizontal rows contain analogous elements, still ordered by increasing atomic weight, one obtains the following arrangement, from which a few general conclusions may be derived.
- The elements, if arranged according to their atomic weights, exhibit a periodicity of properties.
- Chemically analogous elements have either similar atomic weights (Pt, Ir, Os), or weights which increase by equal increments (K, Rb, Cs).
- The arrangement according to atomic weight corresponds to the valence of the element and to a certain extent the difference in chemical behavior, for example Li, Be, B, C, N, O, F.
- The elements distributed most widely in nature have small atomic weights, and all such elements are marked by the distinctness of their behavior. They are, therefore, the representative elements; and so the lightest element H is rightly chosen as the most representative.
- The magnitude of the atomic weight determines the properties of the element…
- One can predict the discovery of many new elements, for example analogues of Si and Al with atomic weights of 65-75…
A few atomic weights will probably require correction…
| Ti=50 | Zr=90 | ?=180 | |||
| V=51 | Nb=94 | Ta=182 | |||
| Cr=52 | Mo=96 | W=186 | |||
| Mn=55 | Rh=104,4 | Pt=197,4 | |||
| Fe=56 | Ru=104,4 | Ir=198 | |||
| Ni=Co=59 | Pd=106,6 | Os=199 | |||
| H=1 | Cu=63,4 | Ag=108 | Hg=200 | ||
| Be=9,4 | Mg=24 | Zn=65,2 | Cd=112 | ||
| B=11 | Al=27,4 | ?=68 | Ur=116 | Au=197? | |
| C=12 | Si=28 | ?=70 | Sn=118 | ||
| N=14 | P=31 | As=75 | Sb=122 | Bi=210? | |
| O=16 | S=32 | Se=79,4 | Te=128? | ||
| F=19 | Cl=35,5 | Br=80 | J=127 | ||
| Li=7 | Na=23 | K=39 | Rb=85,4 | Cs=133 | Tl=204 |
| Ca=40 | Sr=87,6 | Ba=137 | Pb=207 | ||
| ?=45 | Ce=92 | ||||
| ?Er=56 | La=94 | ||||
| ?Yt=60 | Di=95 | ||||
| ?In=75,6 | Th=118? |
2b. Mendeleev: A natural system of the elements and its use in predicting the properties of undiscovered elements (1871).
With the periodic and atomic relations now shown to exist between all the atoms and the properties of their elements, we see the possibility not only of noting the absence of some of them but even of determining, and with great assurance and certainty, the properties of these as yet unknown elements. It is possible to predict their atomic weight, density in the free state or in the form of oxides, acidity or basicity, degree of oxidation, [etc.]. It is even possible also to describe the properties of some compounds of these unknown elements in still greater detail…
Among the ordinary elements, the lack of a number of analogues of boron and aluminum is very striking, that is, in group III, and it is certain that we lack an element of this group immediately following aluminum… The atomic weight of the missing element should be nearly 45. …It should have more basic properties than the lower elements of group III, boron or aluminum, that is, its oxide, R203, should be a stronger base… On the basis of these properties, the oxide of the metal should still be weak, like the weakly basic properties of titanium dioxide; compared to aluminum, this oxide should have a more strongly basic character, and therefore, probably, it should not decompose water, and it should combine with acids and alkalis to form simple salts; ammonia will not dissolve it, but perhaps the hydrate will dissolve weakly in potassium hydroxide… I have decided to give this element the preliminary name of ekaboron, deriving the name from this, that it follows boron as the first element of the even group, and the syllable eka comes from the Sanskrit word meaning “one.” Eb = 45.… The specific gravity of the metal should be close to 3.0, since its atomic weight 45. The metal will be nonvolatile, because all the metals in the even series of all the groups (except group I) are nonvolatile; hence it can hardly be discovered by the ordinary method of spectrum analysis. It should not decompose water at ordinary temperature, but at somewhat raised temperatures it should decompose it, as do many other metals of this series which form basic oxides. Finally, it will dissolve in acids. Its chloride EbCl3 (perhaps Eb2Cl6), should be a volatile substance but a salt, since it corresponds to a basic oxide. Water will act on it as it does on the chlorides of calcium and magnesium… Ekaboron oxide, Eb2O3, should be a nonvolatile substance and probably should not fuse; it should be insoluble in water, because even calcium oxide is very slightly soluble in water, but it will probably dissolve in acid…
This post is available as a PDF here: Newlands, Mendeleev
