Purification of an Unknown Solid by Recrystallization
Purpose:
Recrystallization
- To obtain a pure unknown sample by the means of recrystallization
- To calculate percent recovery through recrystallization
Melting Point
- To identify an unknown by mixed melting point with at least two known compounds
- To identify at least two students having the same unknown
Table of Reagents:
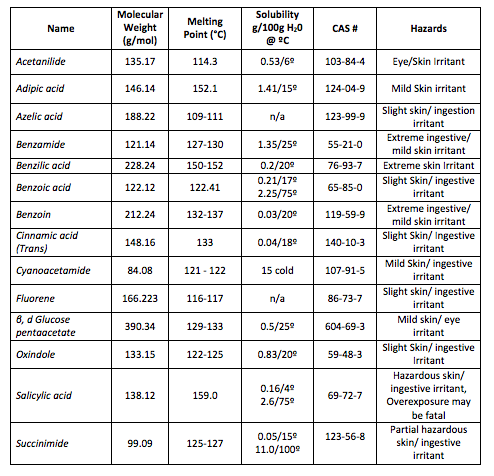
Procedure:
Exp 1A Recrystallization
- Pre-heat a large volume of water to 85-90°C Obtain solid unknown from TA. Record code number.
- Weigh ~2.00g, retain a very small portion for use as a seed crystal later. Record actual mass.
- Add 25mL of water to a 250mL beaker, with stirring bar, on a hot plate.
- Stir and record the temperature (75-80 °C target)
- Add unknown incrementally over 5 minutes and observe solution (grain vs. powder)
- When crystals stop dissolving, add 25mL hot solvent in 3-5mL increments. Record final volume.
- Prepare Vacuum Filtration assembly
- To shut off vacuum, remove vacuum line at filter flask to prevent water back-up into flask
- Warm filter flask, filter and Buchner funnel immediately with hot water before filtration
- This prevents early precipitation and seats filter paper in place.
- Remove water before filtering the hot solution containing the unknown.
- Filter the solution with unknown and check for filtrate clarity
- Transfer the filtrate to a suitable flask for cool down
- Allow to slowly cool to 50 °C on hot plate
- Place the flask in ambient water on the stirrer and cool to 30-35°C
- If no crystals, induce by scratching inside of container with stirring rod.
- Use external ice bath and cool to 0-5°C
- Hold 10-15 minutes at 5°C
- Prepare second Vacuum Filtration assembly
- Vacuum filter the thin product slurry.
- Remove recrystallized wet cake onto a tared watch glass. Record mass of wet-cake.
- Assuming the wet cake in Step 16 is 50% water, calculate an approximate % recovery.
Exp 1B Mixed Melting Points
- Record dry weight of recrystallized dried unknown from Exp 1A
- Mix and crush crystal on watch glass with spatula or test tube
- Prepare Mel-Temp apparatus
- Load 1-2mm into capillary tube and run melting point. Record melting point range
- Select small samples of two pure samples that could be your unknown
- Make mixtures of each with your recrystallized product. Record melting point ranges
- Select two students whose melting points of recrystallized products agree with yours.
- Obtain a small sample of each and run mixed melting points. Record melting point ranges
- Dispose of solids and waste capillaries in appropriate boxes or containers.
Data and Observations:
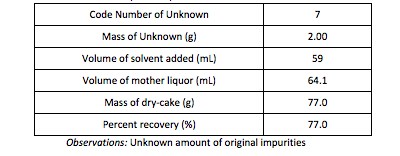
Data Table 1: Summary of Recrystallization Results
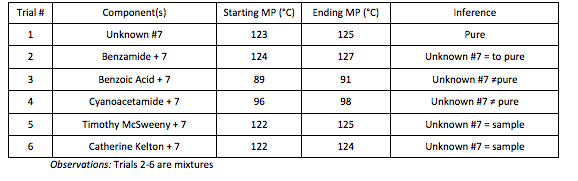
Data Table 2: Summary of Melting Point Results
Results/Discussion:
Part A: From a starting mass of 2.0g of unknown solid, 0.77g (or 77.0%) of Benzamide solid was recovered. It is unknown how much Benzamide solid and unknown impurities were in the sample due to polluted samples. The calculations for the percent of Benzamide recovered with an assumed 1.00g of pure solid are as follows:
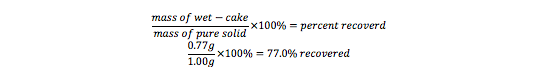
One major error in the recovery of the recrystallized solid was the unknown amount of solid lost due to the spilling of the crystals. During the removal of the crystals after the first hot filtration, one of the Teacher’s Assistants knocked over the Vacuum Filtration Assembly, and the crystals in the Hersh funnel fell onto the table and floor. Most of the solid was recovered, but a large quantity was not recoverable. Other errors in the recovery of the solid were equipment/human error, (5.00%)meaning solids were left as residue on the sides of glassware, the loss of solid in the mother liquor, meaning some of the solid did not recrystallize out of solution due to excess solvent, the transfer from the filter cake to the scale, and random error (5.00%).
Part B: The melting range of the unknown compound was found to be 123-125°C. When mixed with Benzamide, the melting range stayed consistent (124-127°C), while when mixed with Benzoic Acid, the melting range was depressed (89-91°C). After the unknown solid was confirmed to be Benzamide, the compound was mixed with two other students who had also confirmed their solid to be Benzamide. With this additional test, there was no doubt that the unknown was Benzamide.
Conclusions:
The unknown solid (#7) was experimentally found to be Benzamide, with a melting point range from 123-125°C. The other students that were compared to and experimentally found to be Benzamide were Timothy McSweeny and Catherine Kelton.
