Organometallic Chemistry and IR Spectroscopy – Synthesis of Molybdenum Carbonyl Complexes in a Microwave oven and Isomeric Phosphine Complexes
Organometallic Chemistry and IR Spectroscopy – Synthesis of Molybdenum Carbonyl Complexes in a Microwave oven and Isomeric Phosphine Complexes
By: Ansar Kapyatov
Abstract
[Mo(CO)4(NHC5H10)2] complex was prepared using a microwave synthesis method. Then Cis and Trans isomers of [Mo(CO)4(PPh3)2] were prepared and identified using IR spectroscopy and group theory analysis.
Introduction
This experiment is concerned with the study of some organometallic complexes, their thermodynamic stabilities and identification of their isomers.
The actual preparation of our organometallic complexes deserves little attention seeing that the reactions only concern ligand substitutions. The more reactive ligand substitutes the less reactive one and forms a new complex with the metal ion. Perhaps more consideration should be given to carbonyl ligands and the possible geometric isomers being formed in this experiment. IR spectroscopy can distinguish between some isomers and application of group theory can be used to explain and predict different spectra of compounds.
Carbonyl group is a very common ligand in organometallic chemistry and it is exceptionally good at stabilizing low oxidation states. It is useful to consider the ligand in more detail and see its effect on the metal ion in a complex.
Diagram 1: The molecular orbital energy level diagram for CO [1]
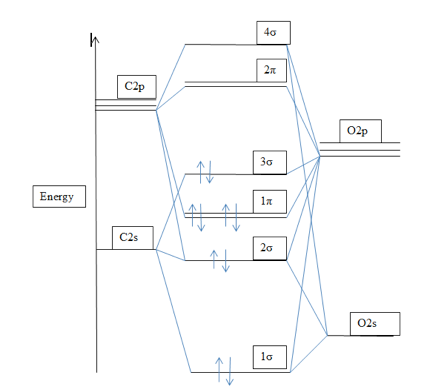
The 1σ orbital is localized mostly on the O atom and is essentially nonbonding. The 2σ orbital is bonding. The 1π orbitals constitute the doubly degenerate pair of bonding π orbitals. The HOMO in CO is 3σ, which predominantly is C2pz in character, largely nonbonding and located on the C atom. The LUMO is doubly degenerate pair of nonbonding π orbitals, with mainly C2p character. In d-metal carbonyls like Mo-CO, the HOMO lone pair orbital of CO participates in the formation of a σ bond and the LUMO antibonding π orbital participates in the formation of π bonds to the metal atom. This type of π bonding is referred to as π backbonding [2]. In practice , the bonding is between Mo – single bond – C – triple bond – O and Mo – double bond – C – double bond – O. Infrared spectroscopy is useful in assessing the extent of π bonding as the CO stretch is easily identifiable seeing that it is both strong and clear of other absorptions.
Most CO stretching bands occur in the range between 2100-1700 cm-1. Both the range of CO stretching frequencies and the number of CO bands are important for making structural inferences [2].
Diagram 2: geometrical isomers of [Mo(CO)4(PPh3)2], where X = PPh3
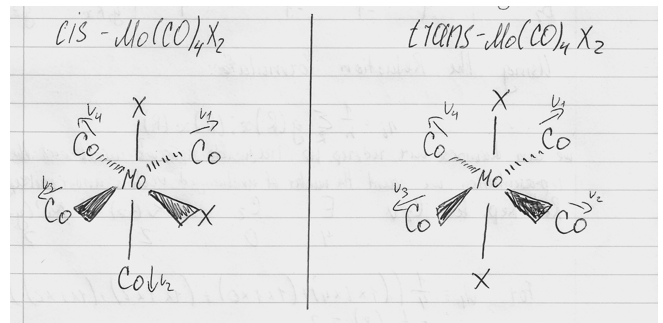
Even without applying any group theory analysis, we can already see from the diagram, that the trans-isomer has a lot more symmetry than the cis-isomer. That fact is confirmed when we see that the symmetry elements of cis [Mo(CO)4(PPh3)2] correspond to the group C2v and elements of trans [Mo(CO)4(PPh3)2] correspond to the group D4h. Now by reducing a representation we can work out the specific symmetry species characters corresponding to each point group. Then, by referring to the character tables we can work out how many modes are IR active. A vibrational mode is IR active, if it has the same symmetry as a component of the electric dipole vector (i.e. the mode will have the same symmetry species as the displacements x,y or z).
Table 1: Character table of C2v point group
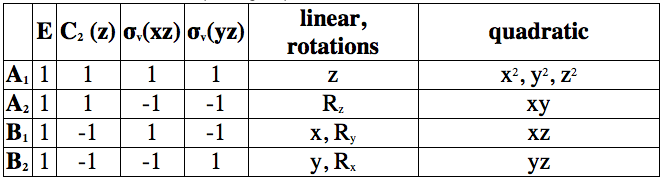
Using the reduction formula we find that our cis – [Mo(CO)4(PPh3)2] complex has 2 A1, 1 B1 and 1 B2 irreducible representations. All 4 are IR active because of the x,y or z characters present in the table. This fact is used to conclude that cis [Mo(CO)4(PPh3)2] isomer will show 4 IR bands in the spectra.
Table 2: Character table of D4h point group
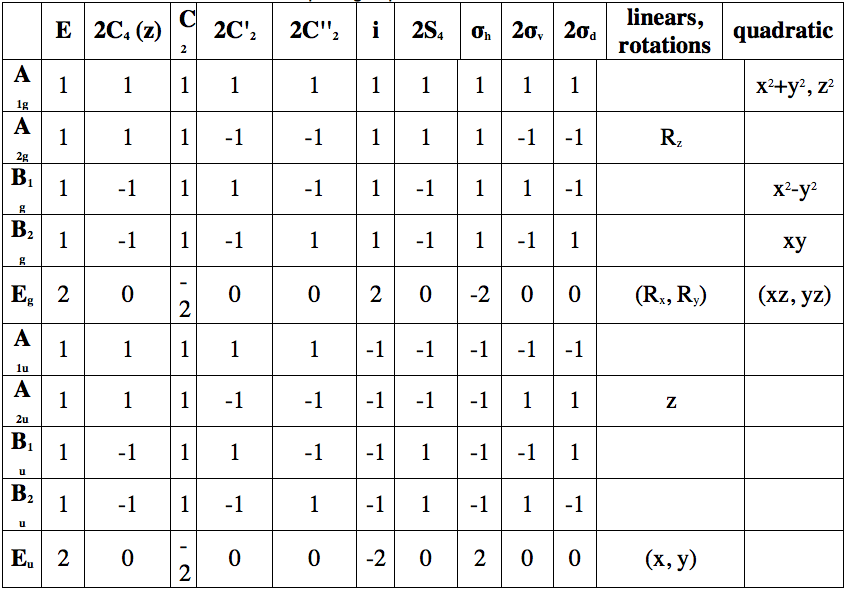
We find that our trans [Mo(CO)4(PPh3)2] complex has A1g, B1g and Eu irreducible representations. Only Eu is IR active and we can therefore predict one peak in our IR spectra of trans [Mo(CO)4(PPh3)2] complex.
There is a high activation energy barrier for isomerisation from cis to trans form. That is why the cis complex is refluxed for a long time. [Mo(CO)4(PPh3)2] complex prefers the cis configuration electronically. However if the activation energy barrier is overcome then the trans isomer becomes the thermodynamic sink and is the most stable compound because of the reduced steric interactions between the non carbonyl ligand groups.
Experimental
1.1 Preparation of the [Mo(CO)4(NHC5H10)2]
7.5mmol of Mo(CO)6 (2.00 ± 0.005g) were mixed with 0.05moles of (C5H10)NH (4.25 ± 0.005g) in THF (5 ± 0.1cm3) and diglyme (12.5 ± 0.1cm3). A pale yellow solution resulted.
3 antibump granules were added to this pale yellow solution.
The solution was connected to an air condenser and put into a microwave to be heated on full power for 40 minutes. Yellow precipitate formed and the solution changed colour from pale yellow to dark brown during that time.
This solution was cooled to room temperature and then filtered through a Hirsch funnel. Yellow solid was washed with hexane (2 x 10 ± 0.1cm3) and left to air dry for 20 minutes. Yield was recorded.
Mass of product was 2.52 ± 0.005 g
Percentage yield of product was 88.8 %
1.2 Preparation of [Mo(CO)4(PPh3)2]
2,5mmol of [Mo(CO)4(NHC5H10)2] were mixed with 5.0mmol of triphenylphosphine in dichloromethane (20 ± 0.1cm3). A pale yellow solution resulted.
3 antibump granules were added to this pale yellow solution.
Solution wats refluxed for 30 minutes on a low heat setting while frequently being swirled. During the firs minute of refluxing the solution changed colour from pale yellow to orange. And in the course of next 29 minutes changed colour from orange to dark orange.
Mixture was cooled to room temperature and filtered under gravity through a filter paper.
Methanol (30 ± 0.1cm3) was added to this dark orange solution. No colour change occurred upon addition.
Solution was cooled in an ice bath to aid crystallisation and then filtered through a Hirsch funnel.
Orange solid was washed with methanol (2 x 10 ± 0.1cm3) and left to air dry for 20 minutes. Yield was recorded.
Mass of product was 0.89 ± 0.005g
Percentage yield of product was 48.6 %
1.3 Isomerisation of [Mo(CO)4(PPh3)2]
Crude [Mo(CO)4(PPh3)2] (0.50 ± 0.005g) were mixed with toluene (20 ± 0.1cm3). A pale yellow solution resulted.
Pale yellow solution was refluxed for 30 minutes. In the first 2 minutes of refluxing the solution has changed colour from pale yellow to orange. Then in the next 2 minutes changed colour from orange to nearly brown. And a dark brown solution resulted after 30 minutes of refluxing.
While the solution was still warm, excess toluene was removed using the rotary evaporator. Dark brown solid formed on the walls of the container upon evaporation of toluene.
This dark brown residue was dissolved in chloroform (30 ± 0.1cm3). Dark brown/grey solution resulted.
This dark brown/grey solution was then filtered by gravity through a filter paper. Dark brown/grey colour stayed on the filter paper while the filtrate was a pale yellow solution.
Methanol (60 ± 0.1cm3) was added to this filtered pale yellow solution. No colour change occurred upon addition.
Solution was cooled in an ice bath for 30 minutes to aid crystallisation and then filtered through a Hirsch funnel. Light yellow solid was washed with methanol (2 x 10 ± 0.1cm3) and left to air dry for 20 minutes. Yield was recorded.
Mass of product was 0.13 ± 0.005g
Percentage yield of product was 26 %
1.4 Recrystallisation of [Mo(CO)4(PPh3)2] isomers
Both isomers were dissolved in minimum volume of chloroform (ca. 3 ± 0.1cm3). Pale yellow solutions resulted in both additions.
Methanol (ca. 6 ± 0.1cm3) was added to resulting solutions. Yellow precipitates started to form in both isomer solutions.
Solutions were cooled in an ice bath for 30 minutes and then filtered through Hirsch funnels. Yellow solids were washed with methanol (2 x 3 ± 0.1cm3) and left to air dry for 20 minutes. Yield was recorded.
Mass of recrystallized product from 1.2 was 0.26 ± 0.005g
Percentage yield of recrystallization was 72.2 %
Mass of recrystallized product from 1.3 was 0.10 ± 0.005g
Percentage yield of recrystallization was 74 %
2 Infra-red Spectroscopy
IR spectra was recorded for all three products between 2200 and 1800 cm3 region using KBr discs.
Very small amounts of samples were mixed with KBr (0.2 g).
Discs were prepared by pressing the mixed samples under a 10 tonne press.
These discs were the put into the spectrometers and the spectra were run.
Calculation of mass of 7.5 mmol of Mo(CO)6:
Relative Molecular Mass of Mo(CO)6= 264.0006 gmol-1
Number of Moles = Mass / Relative Molecular Mass
Mass of Mo(CO)6 to be used = (7.5 x 10-3 mol) x 264.0006 gmol-1 = 1.98 g
Calculation of mass of 0.05 mol of (C5H10)NH:
Relative Molecular Mass of (C5H10)NH = 85.1475 gmol-1
Number of Moles = Mass / Relative Molecular Mass
Mass of (C5H10)NH to be used = (0.05 mol) x 85.1475 gmol-1 = 4.257377 g
Calculation of percentage yield of product from 1.1:
Mass of [Mo(CO)4(NHC5H10)2]obtained = 2.52 ± 0.005 g
Theoretical mass of product = 2.837 g
Percentage yield of product = 2.52 g/ 2.837 g x 100 = 88.8 %
Calculation of mass of 2.5 mmol of [Mo(CO)4(NHC5H10)2]:
Relative Molecular Mass of [Mo(CO)4(NHC5H10)2]= 378.275 gmol-1
Number of Moles = Mass / Relative Molecular Mass
Mass of [Mo(CO)4(NHC5H10)2] to be used = (2.5 x 10-3 mol) x 378.275 gmol-1= 0.9456887 g
Calculation of mass of 5.0 mmol of (C6H5)3P:
Relative Molecular Mass of (C6H5)3P= 262.2857 gmol-1
Number of Moles = Mass / Relative Molecular Mass
Mass of (C6H5)3P to be used = (5.0 x 10-3 mol) x 262.2857 gmol-1 = 1.3114285 g
Calculation of Percentage Yield of product from 1.2:
Mass of [Mo(CO)4(PPh3)2] obtained = 0.89 ± 0.005g
Theoretical mass of product = 1.831 g
Percentage yield of product = 0.89 g/ 1.831 g x 100 = 48.6 %
Calculation of Percentage Yield of product from 1.3:
Mass of [Mo(CO)4(PPh3)2] obtained = 0.13 ± 0.005g
Theoretical mass of product = 0.5 g
Percentage yield of product = 0.13 g/ 0.5 g x 100 = 26 %
Table 3: Infrared peaks from Mo complexes
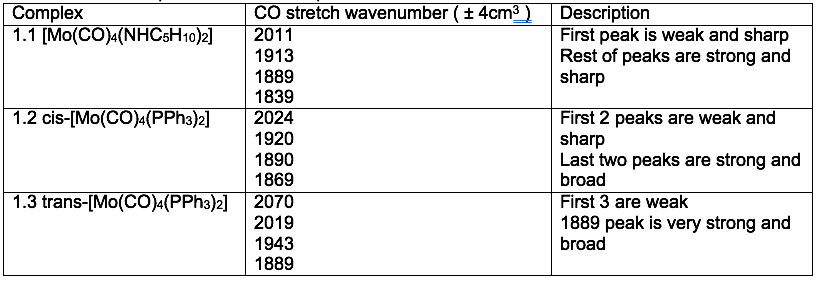
Spectra 1: IR spectra between 2200 and 1800cm-1 of [Mo(CO)4(NHC5H10)2]
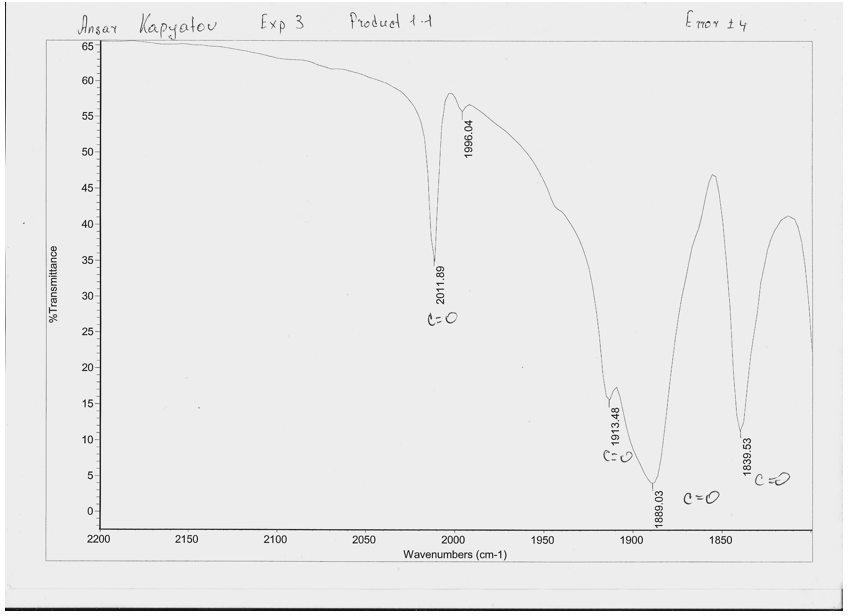
Spectra 2: IR spectra between 2200 and 1800cm-1 of cis-[Mo(CO)4(PPh3)2]
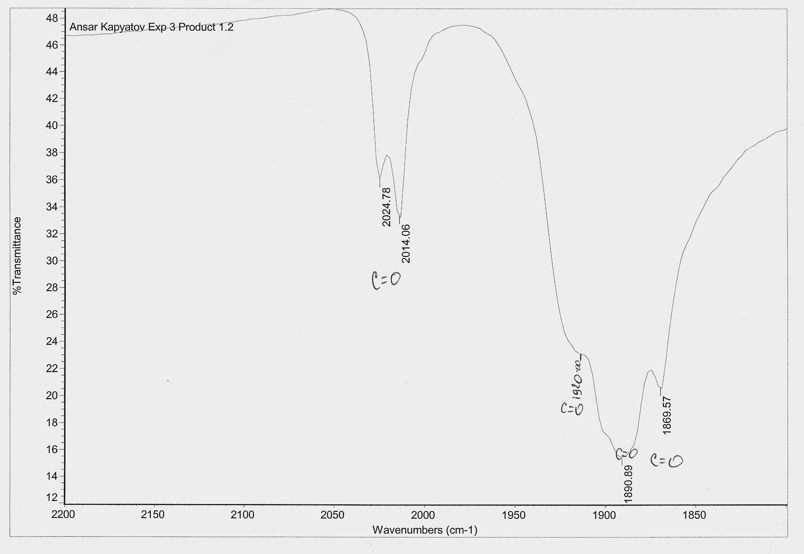
Spectra 3: IR spectra between 2200 and 1800cm-1 of trans-[Mo(CO)4(PPh3)2]
Questions
1.1
A) For the reasons mentioned in the introduction complexes [Mo(CO)4(NHC5H10)2] and cis-[Mo(CO)4(PPh3)2] from experimental 1.1 and 1.2 must adopt the cis geometry (shown in the introduction) because 4 peaks are present on the spectra.
Complex trans-[Mo(CO)4(PPh3)2] from experimental 1.3 must adopt the trans geometry because it has one strong peak band. The other bands shoudn’t in theory be there. They are present in my spectra because the sample could have been concentrated or filled with impurities.
1.2
A) Carbon monoxide adopts π – backbonding interaction of a filled metal orbital and an unfilled ligand orbital. The lone pair of electrons on the carbon is an electron pair donor and the empty antibonding orbital is an electron pair acceptor, this accepts π electron density from the filled d orbitals on the metal atom. This is when the σ bond from the ligand bonds to the metal atom and the π bond from the metal atom bonds to the ligand. The result of this bonding is that the metal – carbon bond is stronger because of the electron density being pushed from the metal atom into the π bond [2].
B) Because my spectra were run in the range of 2200 and 1800cm-1, only carbonyl group peaks are seen on them. C-H bond peaks (2900-3200cm-1) and N-H bond peaks (3200-3400cm-1) will therefore will not bee seen on my spectra. However, the difference between spectra 1 and 2 will be in the presence of N-H peaks in spectra 1. The lone pair on the nitrogen atom allows resonance structures and that results in a lower stretching frequency and therefore peaks with a lower wavenumbers (slightly lower).
References
[1] http://cache.gyazo.com/02402ebcf060e94530d20dbb7b622380.png
[2] Shiver and Atkin’s, Inorganic Chemistry, 5th Edition, OUP, 2010, pp179-196, p 231, p 540, pp 558-560
