Winkler Titration – Boulder Wastewater
Introduction
Oxygen is measured in its dissolved form as dissolved oxygen (DO). Stream systems both produce and consume oxygen. Wastewater from sewage treatment plants such as Boulders often contains organic materials that are decomposed by microorganisms, which use oxygen in the process. If more oxygen is consumed than is produced, dissolved oxygen levels decline and some sensitive animals may move away, weaken, or die.
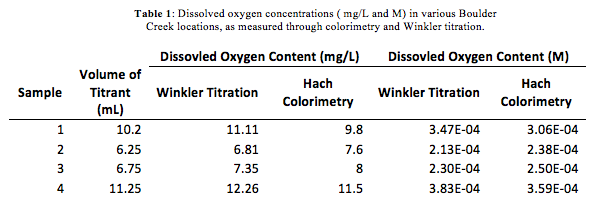
In this lab we took samples from 4 locations. These locations were Boulder Creek upstream, Wastewater Treatment Plant effluent, downstream 200 meters, and downstream a half mile from the mixing point. We first standardized the NaSO4 as a titrant to find the initial concentration then found the DO by a Winkler Titration. We also used AccuVac ampuls to get an accurate reading of DO to compare our titration with. The purpose of this lab was to gain a familiarity with redox chemistry and the Winkler Titration process.
Materials and Methods
In the DO bottles supplied we had 4 samples total, each from a different location on Boulder Creek. We had 1 sample from each of the following locations: 100 m upstream from the treatment plant, the wastewater treatment effluent, 200 m downstream from mixing point, and a half mile downstream from mixing point.
For the standardization of the Sodium Thiosulfate (Na2S2O2 ), we dissolved 2 g potassium iodide solid (KI) into a 250 ml flask, added .3 mL drops of concentrated sulfuric acid (H2SO4) (18 M), and 20.00 mL of potassium bi-iodate solution KH(IO3)2 (0.0021 M). After diluting this to a total of 200 mL we titrated it with Na2S2O3 until the solution turned completely clear and wrote down the volume that was titrated. Using the equation (1), we determined the concentration of S2O32-.
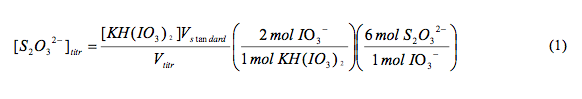
For a relatively accurate measurement of DO we used colorimetric method which involves the Hach DR/890 “pocket colorimeter”. We took a sample of 40mL of Boulder Creek from each of the locations and placed them in 50 mL beakers. Using the High Range(less accurate) DO AccuVac Ampuls we snapped the tops off and allowed to ampules to fill. We shook the ampules for a total of 3 minutes and placed them in the colorimeter for a reading.
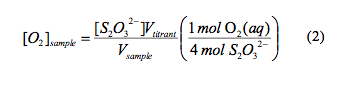
For the Winkler titration we added 1 mL of manganous sulfate solution, and 1 mL of alkali-iodide-azide reagent to the DO bottles of Boulder Creek samples. We waited for the MnO2(s) precipitate to settle at the bottom and added 1 mL of concentrated H2SO4 (18 M) and mixed. When the precipitate dissolved, we added 200 mL of the DO bottle solutions and a stir bar to an Ehrlenmeyer flask. While spinning the solution we titrated with the standardized Na2S2O2 until the solution was completely clear and wrote down the volume that was titrated. We used this process for each sample. The concentration of the DO in each of the samples was determined using equation (2).
Results
Using eqn. 1, it was found that the molar concentration of sodium thiosulfate titrant was 0.027 M. The dissolved oxygen concentrations in the Boulder Creek samples for both the colorimetry and Winkler titration are provided Table 1. The concentrations of DO for the colorimetry were determined using eqn. 2.