ANALYTICAL TECHNIQUES FOR ANALYSIS OF HEAVY METALS
Analytical Techniques for Analysis of Heavy Metals
By: Mohammad Ali Salik
Mohammad Ali Salik
AIM:
The aim of this case study is to choose a suitable instrumental analytical technique to investigate the concentration of heavy metals in sludges collected from contaminated water courses. When choosing the analytical technique, a lot of factors needs to be looked at such as cost, sensitivity ( level of detection), physical state of matrix and availability of instrumentation.
INTRODUCTION:
Heavy metals refer to any metallic chemical element that has a relatively high density and is toxic or poisonous at low concentration. Examples of heavy metals include mercury (Hg), cadmium (Cd), arsenic (As), chromium (Cr), thallium (Tl), and lead (Pb). Higher concentrations of heavy metals can lead to poisoning.
ANALYTICAL TECHNIQUES AVAILABLE:
There are a number of analytical techniques we can use for the determination of heavy metals including:
1. ATOMIC ABSORPTION SPECTROSCOPY (AAS): It relies on the atomization of the determination by flame sources to form free atoms which can absorb radiation at discrete wavelengths. Each wavelength corresponds to only one element and the width of an absorption line is only of the order of a few picometers (pm) which gives the technique its elemental selectivity. The amount of energy absorbed is proportional to the concentration of the determinant present in the sample as the Beer Lambert Law applies.
2. FLAME EMISSION SPECTROSCOPY (FES): It involves the excitation of the sample in a spark or flame to produce line spectra of the elements present. It is a more sensitive technique than flame AAS for some elements & is suitable for multi element analysis.
3. UV / VIS SPECTROSCOPY: It is a relatively fast and simple method for the determination of heavy metals. For the application of this method metals, however, be converted to coloured complexes prior to the analysis using different chemical reagents. This analytical technique is mainly used to obtain quantitative information about a substance. The sample solutions are placed into cuvettes & exposed to UV and/or visible light. The unabsorbed light is then focussed onto a photomultiplier tube detection system using a double beam design. The measured absorbance is proportional to the metal concentration as the Beer Lambert Law applies.
PREFERRED ANALYTICAL TECHNIQUE:
Atomic Absorption Spectroscopy (AAS) is the preferred analytical technique due to the following reasons:
• AAS is most widely used technique which uses simple, inexpensive instrumentation and is rapid.
• AAS can be used to analyse the concentration of over 62 different metals in a solution.
• AAS is very sensitive and can measure down to parts per billion of a gram (ug/dm-3) in a sample.
• AAS can also be used for the analysis of metals like arsenic, antimony, tin, selenium, bismuth and mercury if hydride (generation) AAS is used. It is used to separate and pre-concentrate analytes from sample matrices by a reaction that turns them into their hydride vapours. Sodium borohydride is the common reagent for the reduction.
• FES is not the preferred analytical technique as it has a relatively low precision and suffers from matrix effects & high instrumentation costs.
• UV / Vis spectroscopy is not the preferred analytical technique as although it has a broad area of applicability, high sensitivity and rapid automatic method, BUT the sample preparation is time intensive as well as the measuring procedure (binding to complexes, adjusting pH value, special extraction procedures to obtain coloured metal complexes to be determined by UV / Vis. ALSO it is affected by interferences by other coloured substances in the sample.
ANALYSIS:
For AAS, quantitative analysis can be achieved by measuring the absorbance of a series of solutions of known concentration. A calibration curve and the equation of the line can be used to determine unknown concentration based on its absorbance. All of this can be achieved as the amount of energy absorbed is proportional to the concentration of the determinant present in the sample as the Beer Lambert Law applies:
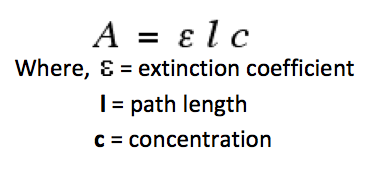
And hence, the absorption (A) is directly proportional to the concentration (c).
AAS makes use of the wavelengths of light specifically absorbed by an element. METAL ions or METALLOIDS in an aqueous sample are vaporised and thermally atomised by a flame. The flame is created by a fuel and oxidising gas. A specific element related spectral light (source: hollow cathode lamp) is radiated through the atomization cell, and a certain wavelength is filtered out of the lines of the spectrum of the element by a monochromator. The difference in the intensity between the sample and reference beams produces a signal which is directly proportional to the total number of free atoms present of the element under analysis. A calibration curve is constructed by running samples of known concentrations under the same conditions as the unknown. With the flame method, a detection limit of a few mg/kg (ppm) is achieved.
APPARATUS:
The block diagram of AAS is as follows:
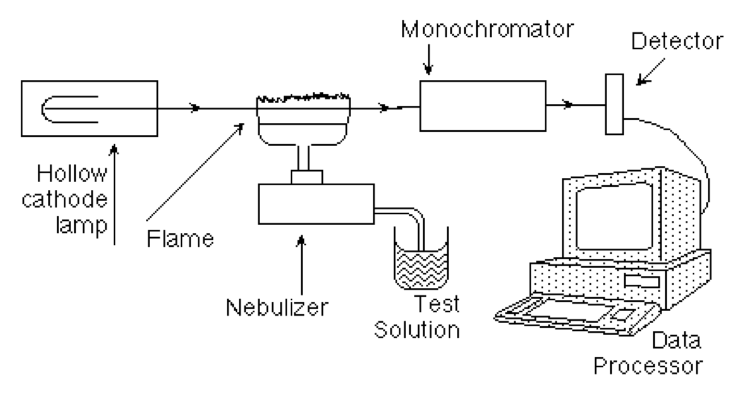
COMPONENTS:
- Hollow cathode lamp: It is most commonly used. The filaments contain elements of interest. The high voltage produces atoms in the excited state and then the electrons fall back to the ground state emitting light of correct wavelength.
- Atomiser: Key component in the instrument which uses temperature as the key factor. There are two types of atomisers:
- Flames:
– Air / methane – low temperature (1900oC) – suitable for easily excited atoms such as Na, K, Li, Ca and Mg.
– Air / acetylene – medium temperature (2300oC) – suitable for most analyses such as Cu, Cr, Ni, Fe, etc.
– Nitrous oxide / acetylene – high temperature (3000oC) – rarely used, required for the most refractory elements such as P.
- Flameless:
– Graphite furnace – Suitable for most elements & allows solids to be analysed as well as solutions.
– Inductively Coupled Plasma (ICP) – wide range of analyses possible & is far more sensitive than flame techniques, but is more expensive and there are also overhead costs.
- Nebuliser: This is another key component. In this process, the sample solution is nebulised (converted into fine aerosol) and introduced to the atomiser.
- Monochromator: This is a diffraction grating system to allow wavelength selection as most elements emit light at more than one wavelength.
- Detector: Usually a photo-multiplier tube. Careful tuning of the detector is necessary to ensure maximum sensitivity. The instrument is “tuned” each time by adjusting the monochromator to maximise the signal.
DISCUSSION:
There are a range of factors which could adversely affect AAS analysis.
- Ionisation interferences: Flame too hot – ions instead of atoms in the flame. This can be overcome if a large excess of an easily ionisable elements such as K, Cs is added which maintains the electron concentrations constant. The substance added is known as “ionization buffer”.
- Spectral interferences: Two elements present in the sample have similar absorption wavelengths. The effects can be avoided by the use of background correction, matrix blank and/or standard addition techniques.
- Chemical Interferences: Formation of thermally stable compounds in the flame can reduce sensitivity. This can be overcome by using a hotter flame and/or adding a releasing agent (a substance which reacts preferentially with the interfering species to form yet another thermally stable compound).
- OPTIMISATION PARAMETERS: These can be divided into 3 groups:
– Source-related parameters
– Atomiser-related parameters
– Monochromator-related parameters
- Effect of lamp current: Source operating power may have a substantial influence upon both absorbance and upon signal-to-noise ratio. For hollow cathode lamp, if the maximum safe operating current is exceeded the lamp may be permanently damaged. Therefore, normally a much lower current around 4-7 mA is used in routine work.
- Choice of Lamp: Multi-element hollow cathode lamps are available for some combination of elements, but they tend to give rather inferior performance to that of single element lamps. If sources such as microwave or electrodeless discharge lamps are employed, these too will have different performance characteristics. When comparing the sources, the absorbance signal size & stability for a suitable dilute standard solution should be compared, with all other conditions carefully optimised & not just lamp intensities and stabilities.
- Choice of Atomiser: It is necessary to choose between flames. Those elements which tend to form thermally stable oxides such as Al, Ti, Si may only be determined in hotter reducing nitrous oxide – acetylene flames. Some elements such as Ba, Cr may be determined in air – acetylene, but are most efficiently atomised in nitrous oxide acetylene flame.
CONCLUSION:
There are a number of analytical techniques we can use for the determination of heavy metals such as Flame emission spectroscopy and UV / Vis spectroscopy, but Atomic Absorption Spectroscopy (AAS) is the preferred analytical technique due to its cost effectiveness, sensitivity and its applicability. Although there are a range of factors which could adversely affect AAS analysis, all these factors can be overcome.
REFERENCES:
• Google images for block diagram of AAS.
• Instrumental techniques: theory and practice book for description of components of AAS.
• http://www.lasalle.edu/~prushan/Intrumental%20Analysis_files/AA-Perkin%20Elmer%20guide%20to%20all!.pdf – for optimization parameters and interferences.
