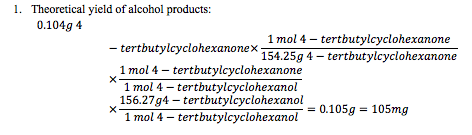Reduction of Ketones Using a Metal Hydride Reagent: 4-tert-Butylcyclohexanol
Written by Breha
Objective: The purpose of this experiment is to reduce ketones, 4-tert-Butylcyclohexanone, to form the alcohol, cis and trans-4-tert-Butylcyclohexanol. The reduction of a ketone carbonyl to alcohol is carried out using sodium borohydride. The alcoholic reactions products are isolated by liquid-liquid extractions techniques and purified by preparative gas chromatography. Cis and Trans diastereoisomers are formed in the reduction of the 4-tert-butylcyclohexanone. TLC was also used to monitor the completion of the experiment.
Experimental Procedure: Experiment 5B was followed as shown in Mayo pages 158-162, with the following modifications to the procedure:
- The reaction was set up on 2X the scale mentioned in the book, meaning that the reaction reagents were doubled
- The progress of the reaction was followed using TLC. Using glass plates 3 spots were created, a standard ketone spot, a cot spot, and a spot using the reaction mixture after 10minutes.
- 1.0 mL of cold 3.0 M HCl was substituted for 1.0 mL of cold 0.1 M HCl.
- The methylene chloride extractions were not transferred to a filter pipet containing anhydrous sodium sulfate instead the typical procedure was used.
- An NMR spectra was not obtained
Reaction scheme:
Data and results:
Data Table:
| Compound | Molecular formula | MW | Amount | mmol | Mp(°C) | Bp(°C) |
| 4-tert-butylcyclohexanone | C10H18O | 154.25 | 104mg | 0.33 | 47-50 | 113-116 |
| Methanol | CH4O | 32.04 | 100µL | -97.6 | 65 | |
| Sodium borohydride reducing solution | 200µL | |||||
| 4-tert-Butylcyclohexanol |
|
156.27 | 105mg | 62-70 | 110-115 |
Observations:
- After the addition of the reducing agent sodium borohydride, the resulting solution was a cloudy white color.
- After the addition of 3MHCl to quench the reaction, bubbles was observed to have form and the reaction solution was a clear color and white precipitate formed on the sides and bottom of the reaction vial.
- The filtered dried organic layer was clear in color. When the dried organic layer was concentrated in a heating block, nothing was observed in the screw cap test tube, however when the concentrate was cooled in an ice bath for 10 minutes, white solid crystals was observed at the bottom of the test tube.
- After the TLC was complete immersed in the p-anisaldehyde stain and cooked on a hot plate, the ketone spot became a dark purple reddish color, while the co-spot separated into three spots, the first spot was an intense light blue color, the second spot was a light greenish color, and the third spot was a dark purple reddish color. The reaction mixture spot separated into only two spots, the first spot was an intense light blue color and the second spot was a small light greenish color.
Calculations:
2. TLC Data:
|
TLC Plate 1:4 Ethyl Acetate: Hexanes |
||||||||||
|
Spot |
Sample |
Solvent Front Distance (mm) |
Spot(s) Distance(mm) |
Rf value |
||||||
|
1 |
Ketone standard |
51 |
33 |
0.61 |
||||||
|
2 |
Co spot(trans/cis/ketone) |
17 |
25 |
35 |
0.33 |
0.49 |
0.68 |
|||
|
3 |
Reaction Mixture (cis/trans product) |
24 |
32 |
0.47 |
0.63 |
|||||
| Spot | Sample | Color |
| 1 | Ketone standard | The spot was a purple redish color |
| 2 | Co-spot | Trans spot was an intense cyan blue color, while the cis spot was a light greenish color and the ketone spot was a purple redish color. |
| 3 | Reaction Mixture | The first spot was an intense light blue color, the second spot was a small greenish color. |
TLC plate representation:
Questions:
- Write a mechanism for the reduction of 4-tert-butylcyclohexanone with sodium borohydride in methanol. Be as complete as possible and show electron flow for all steps. Include the following information:
2. Stereochemistry…
a) The reduction of 4-tert-butylcyclohexanone with sodium borohydride is a stereoselective reaction. What does this mean, in terms of the products that were isolated?
This means that one stereoisomeric product, in this experiment this product is trans-4-Butylcyclohexanol, is preferred over the other. In this experiment, the hydride reducing agent attacks more readily from the axial direction, and the equatorial alcohol is the major product, which is the trans-4-tertbutylcyclohexanol.
b) Can you provide any experimental evidence, from data that you collected during your lab section that supports your answer to part (a)? In other words, do you know if what you expected to happen actually happened in your reaction vial?
The TLC analysis supports this, since the trans-4-tertbutylcyclohexanol had a more intense spot than the cis-4-tertbutylcyclohexanol product, which would indicate that the Trans product had a higher concentration in the reaction mixture.
3. An IR spectrum of a sample of 4-tert-butylcyclohexanol (mixture of isomers) is shown below. Assume that this spectrum was generated from your crude reaction product.
a) What regions of the IR spectrum would you focus on to assess whether or not the desired product 4-tert-butylcyclohexanol was produced from 4-tert-butylcyclohexanone? List a range for each region, in cm-1, and relate each given region to key functional groups in the starting material, product, or both.
- 3299 cm-1 region, the O-H stretching band would be observed, in the product
- 1059-1073cm-1 region the C-O stretching band would be observed in the product
- 2944-2966 cm-1 region the C-H stretching band would be observed in the product and reactant
- In the region 1700cm-1region a C=O stretching band would be observed in the reactant.
b) Based on your answers to parts (a) and (b), comment specifically on the IR spectrum shown below. What does the spectrum tell you about the progress of your reaction?
The spectrum tells us that it belongs to the product, most likely the trans-4-butylcyclohexanol. This is because the C=O stretching band observed in the reactant is absent, and instead an O-H stretching band is observed. We are also able to identify that the IR spectrum belongs to tran-4-tertbutylcyclohexanol because the cis-4-butylcyclohexanol has a more hindered hydroxyl group which adopts some conformations having smaller hydrogen bonded clusters, and as a result the O-H stretching absorption band is therefore split into two.
4. A portion of a 1H NMR spectrum of 4-tert-butylcyclohexanol is shown below, along with a table of chemical shifts, multiplicities, and numbers of protons (from integration) for each peak. Note: the alcohol O-H proton is NOT observed in this spectrum.
a) Is the spectrum consistent with a single isomer, or a mixture of isomers? If a single isomer, which one (cis or Trans)?
This spectrum is consistent with one isomer, the trans-4-butylcyclohexanol isomer.
b) Draw the structure(s) of the correct isomer(s) and circle the key proton or protons that informed your answer to part (a).
c) What peak or peaks in the spectrum correspond to the circled proton or protons in part (b)? Explain your reasoning.
The circle proton corresponds with the H on the axial position next to the alcohol. It would be further downfield, since it is not surrounded by any neighboring hydrogens.
d) Is the absence of any peak or peaks informative? Why or why not?
The absence of the O-H peaks is helpful. It helps identify the product as Trans, since in the cis, the O-H peak would be more pronounced and noticeable.
Work Cited:
Lawlor, M. “Modification for Expt. 5B: Ketone Reduction”; Blackboard Document
Mayo, D. W.; Pike, R. M.; Forbes, D. C. “Microscale Organic Laboratory with Multistep and Multiscale Syntheses”, 5th ed.; John Wiley & Sons, Inc., 2011; pgs. 158-162.
Sigma-Aldrich. “4-tert-Butylcyclohexanol, Mixture of Cis and Trans.” Sigma-Aldrich Co. LLC, 2013.