Synthesis of Ethanol by Simple and Fractional Distillation
Written by Aarti Prabhu
Objective: To purify and concentrate a fermentation mixture by both simple and fractional distillation
|
Procedure |
Observations |
| 1.) Add 5 g of Celite to the fermentation mixture, mix thoroughly, and then vacuum filter into a large filter flask. | Material had settled in the r.b. Flask, but dissolved again when mixed with the Celite. The mixture had a brownish white tint. After filtering, the solution looked yellow and smelt like alcohol. |
| 2.) Transfer the filtered solution back to the 250 mL r.b flask, add boiling chips, and set up for simple distillation. | |
| 3.) The simple distillation requires a heating mantle, variable voltage transformer, distilling flask, 3 way adapter, thermometer, rind stand, condenser, vacuum adapter, and receiving flask. | Drawing of set up below. |
| 4.) Set the voltage transformer on about 30 V while setting up, and then turn to 100 V when ready to begin heating the distilling flask. | |
| 5.) As the mixture begins to boil, drops of liquid will condense on the thermometer, bulb, and distillate will enter the receiving flask. (Graduated cylinder) | The distillate collected in the graduated cylinder at approximately 1 drop every 2-3 seconds. |
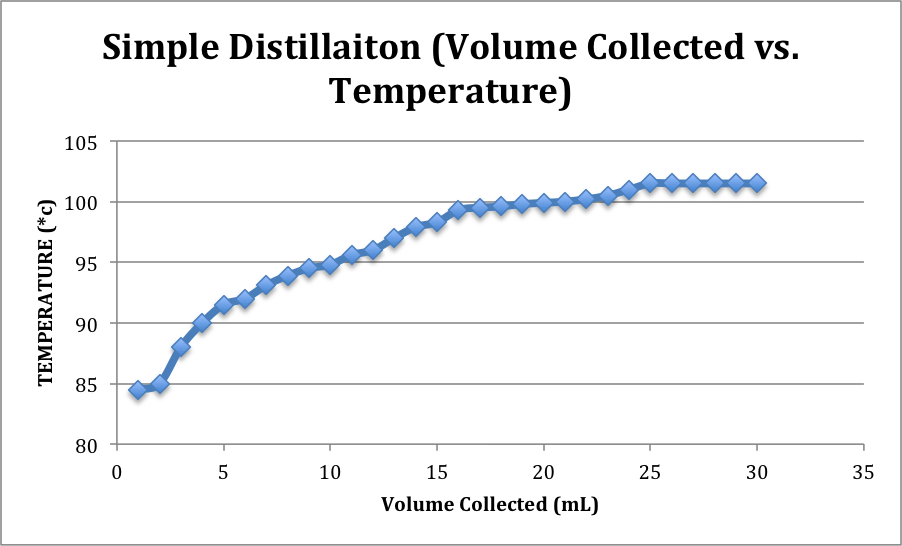
| 6.) Record the temperature at every 1 mL of distillate collected. | Table and graph included below. |
| 7.) Pipet 5.00 mL of the distillate into an already tare 10 mL Erlenmeyer flask. | Mass of empty E. flask: 16.719 g |
Mass of E. flask with 5 mL: 21.412 g
Mass of 5 mL distillate: 4.639 g
Density: 0.9386 g/mL8.) Transfer the 30 mL of distillate to a 100 mL r.b flask, and add boiling chips. Set up for the fractional distillation by adding a fractionating column between the r.b flask, and 3-way adapter.Drawing of set up below.9.) Fill the fractioning column 3/4ths full with glass beads to increase surface area for distillation. 10.) When the mixture boils, a ring condensate vapor will slowly move up the fractionating column, and the distillate should start to collect. 11.) Record the temperature at every 1 mL in the receiving flask. Fraction 1 should be collected 77-80*C. 12.) Fraction 2 is collected from 80*C-96*C. 13.) Fraction 3 is collected from 96*C and up. 14.) Calculate the density of each fraction.
Drawings: Missing 🙁
Data
Simple Distillation:
|
Vol. Collected (mL) |
Temperature (*C) |
|
1 |
84.5 |
|
2 |
85.0 |
|
3 |
88.0 |
|
4 |
90.0 |
|
5 |
91.5 |
|
6 |
92.0 |
|
7 |
93.1 |
|
8 |
93.9 |
|
9 |
94.5 |
|
10 |
94.8 |
|
11 |
95.6 |
|
12 |
96.0 |
|
13 |
97.0 |
|
14 |
97.9 |
|
15 |
98.3 |
|
16 |
99.3 |
|
17 |
99.5 |
|
18 |
99.6 |
|
19 |
99.8 |
|
20 |
99.9 |
|
21 |
100.0 |
|
22 |
100.2 |
|
23 |
100.5 |
|
24 |
101.0 |
|
25 |
101.5 |
|
26 |
101.5 |
|
27 |
101.5 |
|
28 |
101.5 |
|
29 |
101.5 |
|
30 |
101.5 |
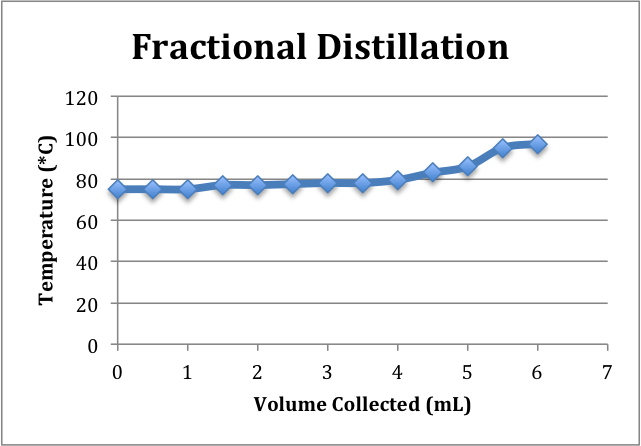
Fractional Distillation:
|
Fractional Distillation |
|
|
Volume of Distillate (mL) |
Temperature Readings (°C) |
|
0 |
75 |
|
0.5 |
75 |
|
1 |
75 |
|
1.5 |
77 |
|
2 |
77 |
|
2.5 |
77.5 |
|
3 |
78 |
|
3.5 |
78 |
|
4 |
79.5 |
|
4.5 |
83 |
|
5 |
86 |
|
5.5 |
95 |
|
6 |
97 |
Fraction #1 (Ethanol):
- Temp range: 77-80C->data points (0-4mL)
- Density: 0.802 g/mL
- % By weight: 95.8%
- % By volume: 97.3%
- 195 proof
Fraction #2 (Azeotrope):
- Temp range: 80-96C->data points (4.5-5.5mL)
- Density: 0.466 g/mL
Fraction #3 (Water):
- Due to unfortunate circumstances when the water pressure increased when the guy across from me turned off his water and caused the hose on my condenser to disconnect and spray water everywhere (getting in my last fraction and disrupting the process so I only collect about 3/4mL of distillate plus about 3mL of just water)
- Temp Range: 96C and above->data points (6mL)
Density: 0.938 g/mL
Results/ Calculations
Simple Distillation:
Mass of empty 10 mL E. Flask: 16.719 g
Mass of E. Flask with 5 mL of distillate: 21.412 g
Mass of distillate: 4.693 g
Density 4.693g/ 5.00 mL = 0.9386 g/mL
%Volume: (0.9386-0.9352) (41.8-47.3) + 47.3 = 45.4 % ethanol by volume 91 proof
(0.9450-0.9352)
% Weight: (0.9386-0.9352) (35.0-40.0) + 40.0 = 38.3 % ethanol by weight
(0.9450-0.9352)
Fractional Distillation:
Mass of empty 10 mL Beaker: 8.901 g
Mass of Beaker with 1 mL of distillate: 9.703 g
Mass of distillate: 0.802 g
Density 0.802 g/ 1.00 mL = 0.802 g/mL
%Volume: (0.802-0.8013) (96.8-97.5) + 97.5 = 97.3 % ethanol by volume 195 proof
(0.8042-0.8013)
% Weight: (0.802-0.8013) (95.0-96.0) + 96.0 = 95.8 % ethanol by weight
(0.8042-0.8013)
Conclusion:
In the mixture of sucrose, yeast, and Pasteur’s salt prepared a week before, fermentation occurred as the enzymes in the yeast converted the sugar to ethanol. The chemical reaction involved in fermentation’s shown below.
As the yeast cells died and the fermentation process stopped, to make a more concentrated substance, the fermented solution was purified by both simple and fractional distillation techniques.
Simple distillation isn’t usually in the whiskey-producing industry, but for the purpose of the lab it was more effective to begin this technique. In the simple distillation set up the distilling flask’s connected directly to the 3-way adapter, which is also connected to the thermometer adapter and condenser. As the fermentation solution is heated to a boil, some of the lower boiling compound, ethanol, escapes into the vapor phase and is condensed again to the liquid phase before collecting into the receiving flask. The rate of collection in the receiving flask was about 1-2 drops per second. The distillation continued until about 30 mL of distillate had collected. The density calculation revealed that the new ethanol concentration was about 45.4 % by volume, 38.3% by weight, which is 91 Proof.
The fractional distillation can further increase the ethanol concentration of the distillate. In the fractional distillation set-up, a fractionating column, filled with glass beads, is placed between the distilling flask, and the three-way adapter. The purpose of the column is to provide additional surface area for the separation of the compounds to occur. The different intervals of the column can be thought of theoretical plates. As the solution boils and vapor enters the column, some will condense upon contact with the glass beads into the distilling flask. The place should reach a state of equilibrium between the vapor and liquid phase for the most effective separation of ethanol from water. Only the compound with the lowest boiling point, ethanol, will reach the top of the column in the vapor phase and become re-condensed in the cooled condenser. However, the ethanol/water solution is an Azeotrope, which means that the two compounds are miscible and boil at a constant temperature. Therefore, impossible to completely isolate the ethanol. The highest concentration of ethanol by volume that can be reached using fractional distillation is 96% which yields 192 proof solution. The density calculation of the fractional distillation collected between 77-80*C yielded a new ethanol concentration of 195 proof/ 97.3% by volume.
Because of time constraints for the fractional distillation not much mL were able to be collected. Also, due to unfortunate circumstances fraction #3 (water) was contaminated by water pressure drop causing hose to spray water into fraction #3.
The difference between the ethanol concentration of the simple distillation product and the first fraction product shows how the fractional distillation was able to increase the ethanol concentration significantly through the use of the column. The fractional distillation was more time consuming than the simple, but provided a more concentrated product. As more ethanol collected in the receiving flask and water remained in distilling flask, the overall distillation temperature would increase before leveling out. This can be seen on the graphs.
Post Lab Questions:
1.) Azeotrope, which means that the two compounds are miscible and boil at a constant temperature. The boiling point of ethanol is lower than water that is why ethanol is the 1st substance to be collected in the distilling flask because it reaches it’s boiling point 1st causing it to turn into the vapor form and condense 1st. The boiling point of our Azeotrope is between ethanol’s boiling point and water’s boiling point. That’s why it’s the 2nd fraction collected.
2.) A pure substance will always boil at a constant boiling point, but constant boiling point doesn’t imply it’s a pure liquid, this is because the substance may be a Azeotrope. An azeotrope’s a mixture with a constant boiling point.
3.) The 1st fraction should contain the highest % volume of ethanol because ethanol has the lowest boiling point so it ends up turning into vapor and condensing before the other substances. The probable composition of the fraction boiling above 96*C is water. The contents of the 2nd and 3rd fraction would probably have higher densities than the 1st fraction, because the concentration of ethanol decreases in the distillate over time.
4.) Minimum boiling Azeotrope is if the Azeotrope (ethanol) boils 1st and after it’s all gone, if there is any other component left, only then will that component distill.
A maximum boiling Azeotrope is if any components come off 1st, and then the Azeotrope. This ethanol-water is an example of a minimum boiling Azeotrope because the ethanol leaves 1st before the other components.