Using the Grignard Reaction to Prepare Triphenylmethanol
Using the Grignard Reaction to Prepare Triphenylmethanol
By: Alexis Huddleston
Abstract
Grignard reagents are considered to be organometallic reagents and are therefore usually strong Lewis bases and function as good nucleophiles. In anhydrous reaction conditions, the formation of Grignard reagents can occur when the reagent is reacted with an organic halide. As demonstrated in the lab, phenylmagnesium bromide, an organic halide, can react with methyl benzoate along with the Grignard reagent to produce triphenylmethanol. Since Grignard reagents are extremely reactive with Lewis acids such as water, precautions were taken during the lab to ensure that the reaction was not ruined due to the presence of water (Organic Chemistry I and II, 89). The phenylmagnesium bromide was prepared in order to begin the reaction and was added to the methyl benzoate. The melting point of the resulting product of the Grignard reaction allowed the purity of the product to be evaluated. Additionally, the IR peaks present in the IR spectrum of the product allowed the functional groups of the triphenylmethanol to be observed. Notably, the absence of water during the Grignard reaction allowed for the product of the reaction, triphenylmethanol, to be synthesized successfully.
Introduction
The use of Grignard reagents to produce compounds such as triphenylmethanol has been found to be not only a useful method of synthesizing an extended variety of functional groups but also to be a versatile technique. Interestingly, in modern industry, the application of Grignard reactions of large scale has decreased over time because of the cost inefficiency. Additionally, the potential dangers of using an extensive amount of diethyl ether, a solvent used in this experiment, also affected the decline of industrial applications of Grignard reactions (Teixeira et. al. 714-715). Yet, in order to explore the method in which phenylmagnesium bromide and methyl benzoate can react to produce triphenylmethanol, the Grignard reaction was utilized during this experiment.
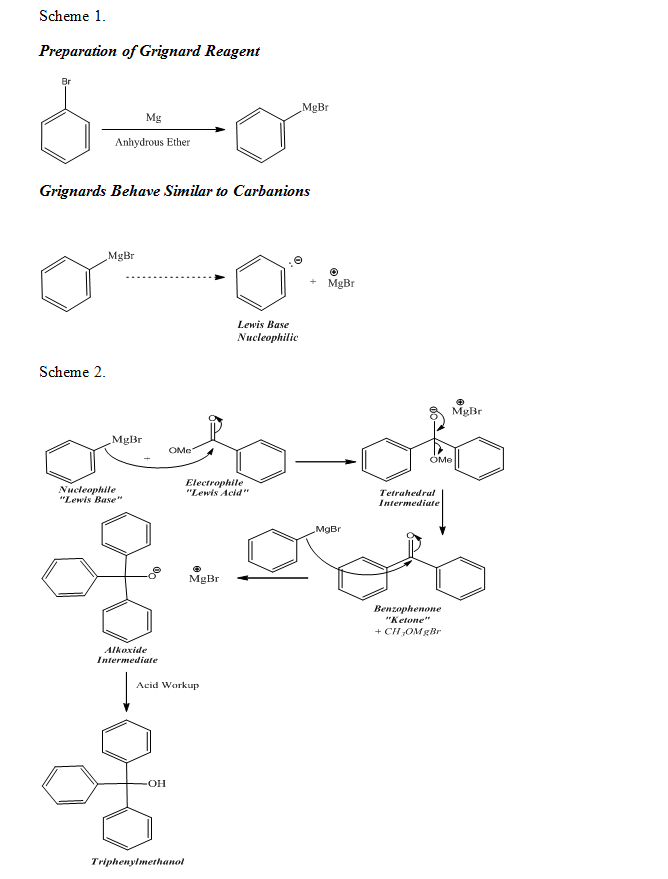
The synthesis of a Grignard reagent was required for the Grignard reaction of this experiment to occur, therefore, phenylmagnesium bromide was produced before further proceeding with the preparation of triphenylmethanol. Grignard reagents, such as phenylmagnesium bromide, are Lewis bases and are good nucleophiles; they are also good leaving groups. Due to the increase of the size of a molecule upon descending down the periodic table, it was evident that phenylmagnesium bromide was a good leaving. Additionally, the continuation of the Grignard reaction in producing the final product was due to the fact that ketones are more reactive with nucleophiles than esters. Consequently, the ketone, benzophenone, was able to utilize the Grignard reagent to further the Grignard reaction to completion, thus producing triphenylmethanol, the final product
As shown by the mechanisms below which represent the chemical reactions that occurred during the experiment, the preparation of the Grignard reagent was the initial step of a Grignard reaction. Also, as seen in ‘Scheme 2’ below, the Grignard reagent was used to produce the final product, triphenylmethanol (Organic Chemistry I and II, 90). Upon synthesizing the final product, the melting point of the product was observed and recorded, allowing the purity of the product to be evaluated. Additionally, an IR spectrum of the product was obtained and analyzed for specific peaks, thus allowing the further assessment of the purity of the product. The results of the lab thoroughly illustrate the effects of properly utilizing the Grignard reaction, specifically in the production of triphenylmethanol.
Materials and Methods
Materials:
- Aluminum foil
- Needles
- 1.0 mL syringes
- Bromobenzene
- Diethyl ether
- Methyl benzoate
- Microscale apparatus
- 3 mL conical vials
- 10 mL round bottom flask
- Spin vane
- Magnesium turnings
- Spatula
- Iodine crystals
- Cap and septum
- Hot plate
- Small beaker
- Ice water
- Fume hood
- Distilled water
- 3M HCl solution
- Filter-tip pipette
- 5 mL conical vials
- 50 mL Erlenmyer flask
- Saturated NaCl solution
Methods:
Step 1: Before beginning the experiment, wrap the glassware involved in aluminum foil and place the glassware in an oven to ensure that the glassware is dry and free of water.
Step 2: Obtain the glassware from the oven and unwrap the aluminum foil.
Step 3: Get one needle and three 1.0 mL syringes. One syringe will be used for bromobenzene, one for methyl benzoate, and one for adding diethyl ether to the reaction. The syringe used to add diethyl ether to the reaction will be used throughout the experiment.
Formation of phenyl magnesium bromide:
Step 1: Assemble the microscale apparatus to be used during the preparation of phenyl magnesium bromide.
Step 2: Label two clean, dry 3 mL conical vials “vial #1” and “vial #2”. Before proceeding to the next step, remember to quickly recap all of the reagents used in the experiment to ensure that there is no contamination from water vapor.
Step 3: Into vial #1, place 2.5 mmol bromobenzene and 1.5 mL diethyl ether and immediately seal the mixture using a cap and septum. Gently shake the mixture.
Step 4: Into vial #2, place 1.00 mmol (136.1 mg, 0.1361g) methyl benzoate and 1.5 mL diethyl ether and immediately seal the mixture using a cap and septum. Gently shake the mixture.
Step 5: Place into a 10 mL round-bottom flask equipped with a spin vane, 2.1 mmol magnesium turnings and 1.0 mL of diethyl ether.
Step 6: Use a spatula to add a small piece of iodine crystal through the opening labeled “cap A” on the apparatus.
Step 7: Using a 1.0 mL syringe, add 1.0 mL of diethyl ether through the septum of “cap A” to rinse the iodine down into the flask.
Step 8: Set the temperature of the hot plate to approximately 90⁰C and reflux the solution in the round bottom flask while stirring for approximately 5 minutes.
Step 9: Using one of the 1.0 mL syringes, transfer 4-5 drops of the reagent mixture from vial #1 through the septum of “cap A”. Continue refluxing for an additional 3 minutes.
Step 10: Transfer the remaining contents of vial #1 into the round bottom flask over a 10-minute period.
Step 11: Continue refluxing for approximately 10-15 minutes or until nearly all of the magnesium reacts and the solution turns a clear amber color.
Formation of triphenylmethanol-the product:
Step 1: For a 2-minute period, use a second clean syringe to transfer all of the reagent contents of vial #2 through the septum of “cap A” into the round bottom flask. Continue refluxing during the 2-minute period.
Step 2: Reflux the solution for an additional 30 minutes.
Step 3: While keeping the round-bottom flask capped and sealed, allow the solution to cool to room temperature using an ice water bath. Use a small beaker for the ice water bath and place the round-bottom flask inside the beaker so that it can be cooled.
Step 4: Place the round-bottom flask into a fume hood and carefully remove “cap A.”
Step 5: Into the round-bottom flask, add 20 drops of distilled H2O dropwise. Begin swirling the solution. Continuing swirling, add drops of a 3M HCl solution to the reaction. If necessary, add more diethyl ether to the solution.
Step 6: Observe the evolution of the H2 gas during the reaction and then remove the reaction mixture from the ice water bath.
Step 7: Disassemble the microscale apparatus.
Step 8: Observe the biphasic clear mixture of organic diethyl ether layer at the top and the acidic aqueous layer at the bottom of the reaction mixture.
Isolation and characterization of the product-Triphenylmethanol via extraction:
Step 1: Using a clean filter-tip pipette, transfer the biphasic mixture from the round-bottom flask to a clean, dry 5 mL conical vial.
Step 2: Rinse the round-bottom flask with a small amount of diethyl ether. Transfer the rinse solution from the round-bottom flask into the 5 mL conical vial used in the previous step.
Step 3: Use forceps or tweezers to remove the spin vane from the round-bottom flask and place the spin vane into the 5 mL conical vial carefully, avoiding excessive splashing or loss of solution.
Step 4: Use a micropipette to carefully remove the aqueous layer of the solution and transfer it to a clean, dry 50 mL Erlenmyer flask and set it aside.
Step 5: Into the remaining diethyl ether organic solution in the 5 mL conical vial, add 1-2 mL of distilled water and allow the solution to stir for approximately one minute.
Step 6: Use a pipette to remove the second aqueous layer. Repeat the rinsing process if necessary. Combine these rinses with the initial rinse within the 50 mL Erlenmyer flask.
Step 7: Add 1 mL of a saturated NaCl solution to the 5 mL conical vial containing the diethyl ether organic layer as a final rinse. Vigorously stir the solution in the 5 mL conical vial and remove the aqueous layer. Combine this rinse with the other rinses in the 50 mL Erlenmyer flask.
Step 8: Dry the diethyl ether organic layer with MgSO4 (magnesium sulfate).
Step 9: Get a vial and cap, weigh the vial with the cap on it, and record the weight.
Step 10: Get a glass pipette and pack it with a small amount of cotton and silica gel.
Step 11: Use a clean filter-tip pipette to add the diethyl ether organic layer and MgSO4 mixture to the glass pipette to be filtered into the vial. After the mixture has been completely filtered, tightly seal the vial with its cap and allow the product to dry at room temperature.
Step 12: Make sure that the product is completely dried. Weigh the vial while still tightly sealed with the cap and with the product inside it; record the weight.
Step 13: Calculate the percent yield of the product.
Step 14: Obtain an IR spectrum of the product and analyze the peaks.
Step 15: Clean all glassware and the experimental area.
Results: Data and Calculations
- weight of vial + cap = 16.2635g
- weight of vial + cap + product = 16.3284g
- actual yield of product = 16.3284g – 16.2635g = 0.0649g
- theoretical yield of product = amount of methyl benzoate used (g)/ molecular weight of methyl benzoate x molecular weight of triphenylmethanol = 0.1365g/ 136.15 x 260.33 = Yg = 0.2609g
- percent yield = product (g)/ Yg = 0.0649g/ 0.2609g x 100% = yield = 24.87%
- melting point range: 163.7⁰C – 165.8⁰C
IR Spectrum:
- most peaks in fingerprint region
- Peak at 3473.99 cm-1 represents an alcohol
- Peak at 3060.58 cm-1 represents a carbon to hydrogen stretch (single bonded)
- Peak at 1959.21 cm-1 represents a carbon to carbon stretch (double bonded)
- Peak at 1596.90 cm-1 represents a carbon to carbon stretch (double bonded)
Discussion/ Conclusion
During the lab, a reaction of phenylmagnesium bromide with methyl benzoate was conducted to produce triphenylmethanol. Specific precautions, such as completely drying equipment and tightly sealing vials quickly were taken to ensure that the Grignard reaction was free of water. As a result, there was an ample amount of triphenylmethanol produced as a final product of the Grignard reaction. During the formation of the product, numerous color changes and chemical reactions were noticed, including the formation of bubbles when distilled water was added to the reaction mixture indicating that magnesium was being dissolved. Also, a color change was noticed in the reaction mixture after the amber colored solution was produced during the formation of the phenylmagnesium bromide. Once the contents of vial #2, which consisted of 1.00 mmol (136.5 mg, 0.1361 g) of methyl benzoate and 1.5 mL of diethyl ether, from the formation of the phenylmagnesium bromide was applied to the round bottom flask, a variety of color changes were noticed. The amber colored solution became a darker brown, then changed to a pink color, and finally changed to a peach color. Upon the completion of the formation of triphenylmethanol, the reaction mixture had two layers; the top layer was the product.
The color of the resulting liquid product was observed after completing the filtration of the product; the color was a dark orange. After the product was allowed to dry, the product obtained a peach orange color. When weighed, the triphenylmethanol which was produced was 0.0649g. Also, upon obtaining the melting point of the product, it was observed that the melting point range of the product was 163.7C -165.8C. Since the melting point range of the product was close to the standard melting point of triphenylmethanol, 160⁰C, it was determined that the product must be considerably pure. When analyzing the peaks of the IR spectrum that was obtained of the product, the various IR peaks were able to be deciphered and the functional groups that were present in the product were determined. The IR spectrum showed specific peaks at 3473.99 cm-1 which indicated the presence of alcohol, 3060.58 cm-1 which represented a carbon to hydrogen stretch, 1959.21 cm-1 which represented a carbon to carbon stretch, and 1596.90 cm-1 which represented a carbon to carbon stretch and was also indicative of aromatic bending. Triphenylmethanol consists of an alcohol group and aromatic bending; according to the peaks in the IR spectrum, the product that was synthesized during the experiment possessed specific functional groups that are present in the structure of triphenylmethanol. Therefore it is plausible to conclude that the production of the triphenylmethanol was successful. Also, I was able to determine that the percent yield of the product was 24.87%, which indicated that an acceptable yield of triphenylmethanol was synthesized during the experiment.
Though the results of the experiment illustrate the effectiveness of the Grignard reaction in producing triphenylmethanol, the yield of the product could have increased. During the experiment, there could have been more precaution taken to ensure that the reaction was free of water. It is possible that during the process of assembling the microscale apparatus or mixing chemical to form the various reaction mixtures that water vapor was able to settle along the microscale apparatus, thus affecting the production of the triphenylmethanol. In the event that water was able to settle along the apparatus, the yield of triphenylmethanol available after the completion of the Grignard reaction was probably less than expected. Precautions such as mixing all of the reaction mixtures necessary for the experiment and capping them before assembling the microscale apparatus could help to reduce the possibility for traces of water to enter into the Grignard reaction and negatively affect the production of triphenylmethanol. Thus, it is necessary to apply an extended amount of precaution and effectively keep the reaction free of water; this will most likely result in an increased yield of product and increased accuracy in results.
Literature Cited
Teixeira, Jennifer M., et al. “The question-driven laboratory exercise: a new pedagogy applied to a green modification of Grignard reagent formation and reaction.” Journal of Chemical Education 87.7 (2010): 714-716.
Masterson, Douglas and Tina Masterson. Organic Chemistry I and II. Dubuque: Kendall Hunt Publishing Company, 2010. Print.
