Wittig Synthesis of Alkenes
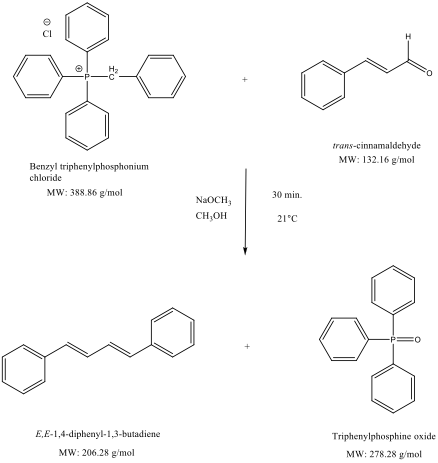
Purpose: To synthesize E,E-1,4-diphenyl-1,3-butadiene via Wittig reaction (Wittig Synthesis) using Benzyl triphenylphosphonium chloride and cinnamaldehyde in sodium methoxide.
Reaction Equation(s):2
Procedure:2
A suspension of anhydrous methanol (1.0 mL), benzyl triphenylphosphonium chloride (0.7508 g, 1.9308 mmol), cinnamaldehyde (0.2415 g, 1.8273 mmol, 0.23 mL) and sodium methoxide (2.4 mL, 4.8 mmol, 2M) was stirred at 21˚C for 30 min. The crude product was isolated via semi-microscale vacuum filtration for 15 min. and rinsed with cold methanol and water. The isolated, crude product was recrystallized in hot methanol and isolated via semi-microscale vacuum filtration to obtain E,E-1,4-diphenyl-1,3-butadiene (0.0841 g, 0.4077 mmol, 22%) as a white, crystalline solid with mp 150-151˚C (mp lit3 150-152˚C).
Discussion:1
Benzyl triphenylphosphonium chloride and cinnamaldehyde were reacted with sodium methoxide to obtain E,E-1,4-diphenyl-1,3-butadiene as a white, crystalline solid in 22% yield. The melting point of the crystal product was determined to be 150-151˚C, which was within the literature range, thus supporting the identity of the compound. Resulting in such a small range suggests that the final product was safe from contaminations of unreacted starting materials, solvents, or by-products. The yield was low and could be improved with the following considerations in mind: when the ylide is formed, its zwitterionic form can become very unstable and reactive with other molecules than the chosen carbonyl compound. This ability to stabilize as a zwitterion can also lead to other polar reactions with contaminants in the reaction vessel. Not only the ylide can perform such reactions but cinnamaldehyde is an α,β-unsaturated ketone and thus has the potential to form an enolate, reacting with sodium methoxide, instead of elimination taking place on the triphenylphosphonium chloride group. According to analysis by Adlercreutz and Magnusson, “competitive enolate formation is the main reason for the low yields of olefin sometimes encountered in Wittig reactions between ketones and methylenetriphenyl-phosphorane.” Enolates can be saved by adding water to the mixture post-ketone addition, quenching the enolates to produce more reactant for the ylide to come in contact with. Through several cycles of extraction, drying, olefination and quenching, the product alkene yield will increase while the enolate concentration will diminish to almost none being reacted.1 Thus, according to Le Chatelier’s principle; the reaction will be pushed to the right as reactants and water are added.
References:
(1) Adlercreutz, P.; Magnusson, G. Synthetic and Mechanistic Aspects on the Wittig Reaction. A
Yield Increasing Modification. Acta Chemica Scandinavica 1980. 34, 647-651.
(2) Callam, C.; Paul, N. Organic Chemistry Lab – Chemistry 2550; McGraw-Hill: New York,
2012; pp. 130-133.
(3) Sigma-Aldrich Chemical Catalog Online. http://www.sigmaaldrich.com/united-states.html
(accessed Jan-Feb 2013); search terms: cinnamaldehyde, trans,trans-1,4-diphenyl-1,3
butadiene.